You don't need to be a rocket scientist to know a little about...rocket science.
Imagine the perfect rocket fuel. When "detonated," it would deliver colossal thrust, propel a spacecraft out of Earth’s atmosphere, and do so without the nasty baggage: no toxic hydrazine, no heavy metals, no combustion byproducts, and no fires. A dream, right? Right now, it is. But some jaw-dropping research out of Germany has the possibility of revolutionizing rocket propulsion. With a few caveats.
I’ve written before about the science behind what makes things go boom, in a piece cleverly titled Why Stuff Explodes. It attracted a lively (read: not quite sane) readership, perhaps from the fringes of the internet, some of whom seemed a little too excited about things that blow up. Some of them may even possess an even number of chromosomes, but I'm not one to judge [1].
The chemistry of explosions, though, is straightforward: compounds rich in nitrogen and oxygen, but poor in carbon, are nature’s ticking time bombs. The less carbon, the cleaner the burn. The more nitrogen and oxygen, the bigger the bang.
Enter hexanitrogen — a molecule composed entirely of nitrogen atoms, which was just published in a paper in the journal Nature by Peter R. Schreiner and colleagues, at the Institute of Organic Chemistry, Justus Liebig University Giessen. There has never been anything like it. The only neutral [2] molecule that contains only nitrogen is nitrogen gas (aka dinitrogen, N2), an inert gas which makes up 78% of the atmosphere.
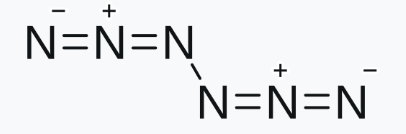
The chemical structure of hexanitrogen.
Hexanitrogen doesn’t just defy chemical convention — it stores an astonishing amount of potential energy in its strained nitrogen-nitrogen bonds. When released, that energy transforms into nothing but pure nitrogen gas (N₂), meaning: enormous thrust, zero pollution. This molecule is arguably one of the most exotic and energetic substances ever created in a lab.
Before we get into the (very cool) chemistry, let's look at a section of the paper that absolutely no one on earth will understand. This includes me.

Anything that begins with "According to CCSD(T)/cc-pVTZ (ΔH0) computations" falls under the umbrella of physical chemistry, specifically thermodynamics, a subspecialty so
beyond comprehension that it makes organic chemistry seem like playing with Legos.
Why would I expose anyone to that indecipherable mess above? The info I underlined in green is why. It's telling us that hexanitrogen is a more powerful explosive than some of the bad ass monsters that are in use (Figure 1).
Some bad ass monsters

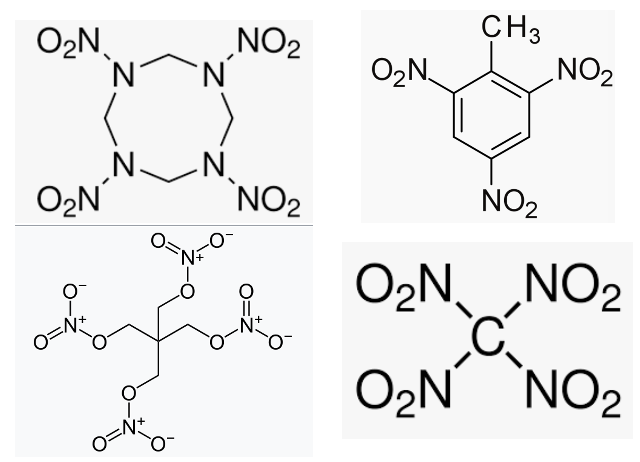
Figure 1 (Top): Four of the most demonic explosives, their oxygen + nitrogen content, and some vaguely disturbing comments.
Back to hexanitrogen
Hexanitrogen has made a very big splash in the explosive world of explosives:
‘"It would be the most useful rocket fuel on the planet. N6 would not burn with a flame: it’s just a burst of energy that generates a large volume [of gas] – so a lot of thrust, and it is non-corrosive...This work is spectacular and, in my opinion, is worthy of a Nobel prize."
Karl Christe, aka 'The Fluorine God,' University of Southern California, in Chemistry World
Not a garage experiment
Nothing is as easy as it seems, and making hexanitrogen is rather different than Jiffy Pop.
- The chemicals involved in making it are some real doozies.
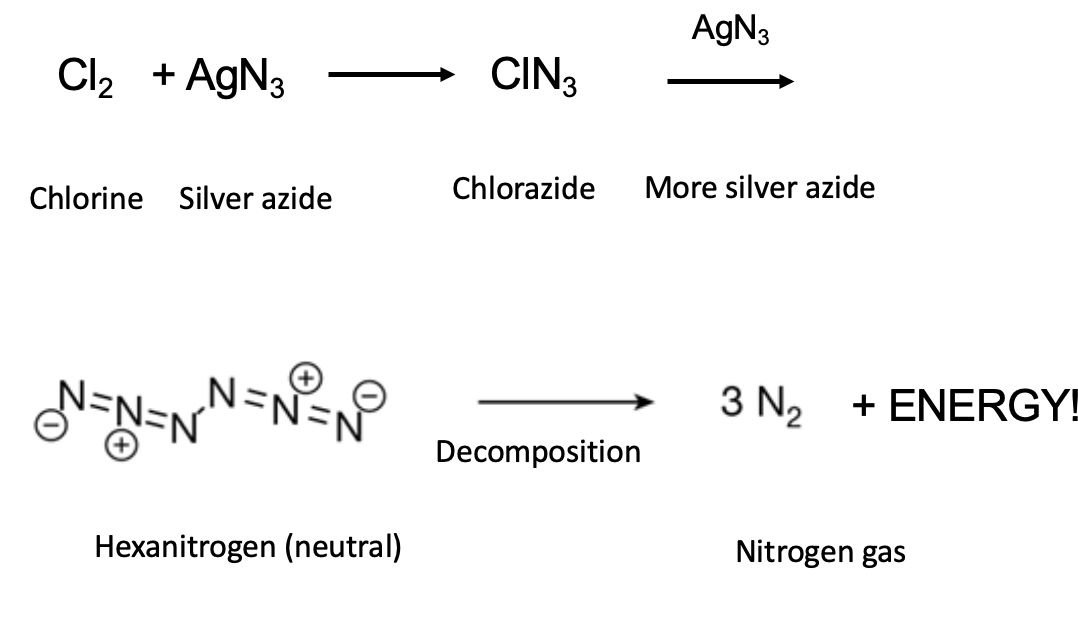
Chlorine, which was used as a chemical weapon in WWI, is something to avoid. One good snootful and a cacophony of things will happen to you, ranging from very bad to worse. Silver azide is a violent contact explosive; it can detonate (when dry) simply by putting a beaker or flask on the same table. The vibrations are sufficient to set it off.
But note the final step. When hexanitrogen decomposes, it gives off nothing other than non-toxic, non-polluting N2, making it a perfect fuel. Of course, turning a high-energy explosive made on a milligram scale in a lab is still a long way from becoming a useful, controllable propellant. This is no small feat that remains unsolved.
Now you have it, now you don't
OK, so now you've managed to make hexanitrogen without offing yourself. There is a minor problem – stability. The half-life of the stuff is 0.036 seconds at room temperature, meaning that in 0.36 seconds, 99.9% of it will be gone. That sounds like a lot of work for nothing. But it isn't. When cooled in widely used liquid nitrogen (-321°F), the group calculated its half-life to be >100 years. Quite the difference, and not a big deal logistically. A number of currently used rocket fuels have to be stored in liquid nitrogen.
Bottom line
This discovery has the potential to be a game-changer in the world of rocket propulsion, although there are still issues to solve.
Philipp Wagner at the University of Tübingen in Germany sums it up:
"I think the most remarkable thing is that they made this molecule at all,’ he says. ‘Whether it will have any applications or not remains to be seen.’



