
The latest controversy regarding messenger RNA persisting in the body, potentially causing harm from COVID-19 vaccines, should be settled. Or is it?
Matt Herper’s new piece in Stat tackles the issue head-on with an interview with one of the co-inventors of the mRNA vaccines for COVID.
The Controversy
Discounting those who are simply anti-vaccine, some credible-sounding individuals still argue there’s an inherent safety issue with the Moderna and Pfizer vaccines because the mRNA supposedly persists. One of the most prominent is Dr. Retsef Levi, who now chairs ACIP’s working group on COVID-19 vaccines. As he put it:
“The initial safety paradigm was that the vaccine contents would only stay in the arm and be cleared after a short duration. Now we know that’s not true – so we need to understand the biodistribution and persistence of the mRNA, the spike protein, and the lipid nanoparticles, and what their respective risks are.”
— Retsef Levi, Brownstone.org
A Nobel Prize Winner Disagrees
Herper’s article contrasts these claims with the views of Dr. Drew Weissman, Professor of Medicine at the Perelman School of Medicine, University of Pennsylvania, and co-laureate of the 2023 Nobel Prize in Physiology or Medicine for developing mRNA vaccines.
Asked whether vaccine mRNA could persist for months in a rare patient, Weissman was unequivocal:
“It is absolutely impossible. mRNA is degraded incredibly rapidly. When you modify it, it’s a little slower. It’ll last 24 hours. It never, ever lasts six months. That’s just impossible.”
Why Chemistry Sides With Weissman
RNA is, by its very nature, a short-lived messenger, while DNA is built for stability and long-term storage. This isn’t speculation—it’s basic biology and chemistry. RNA’s fragility is exactly why cells use it for temporary instructions and DNA for the permanent archive. That simple fact, reinforced by both chemistry and biology, is why claims of vaccine mRNA persisting for months or years don’t hold up.
The Chemistry Behind RNA Instability
Behind all the noise is a fundamental, well-known chemical reaction called ester hydrolysis – the breaking of an ester bond by water, producing a carboxylic acid and an alcohol. (Figure 1) While this may seem irrelevant to vaccines, it is anything but.
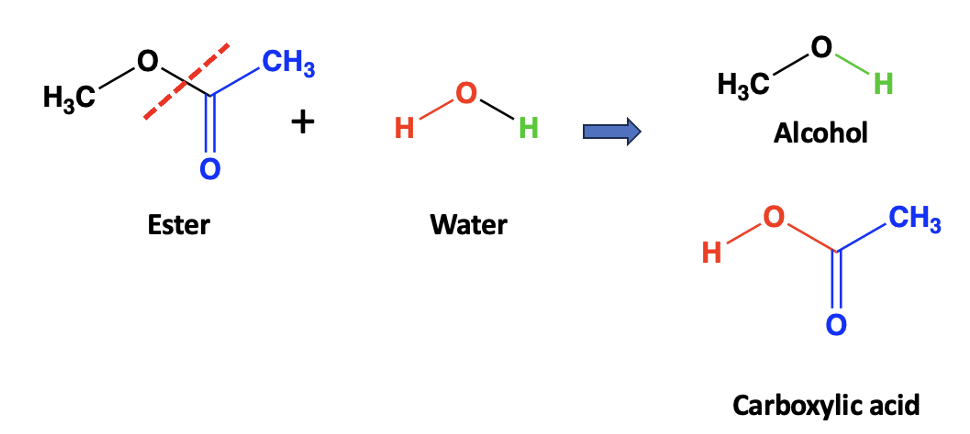
Figure 1. Hydrolysis of an ester involves the addition of water, followed by the breaking of the ester bond (red hatched line), which forms its components – an alcohol and a carboxylic acid.
Phosphate esters, the backbone of DNA and RNA, can also be hydrolyzed. The process (Figure 2) is conceptually identical to that of esters.
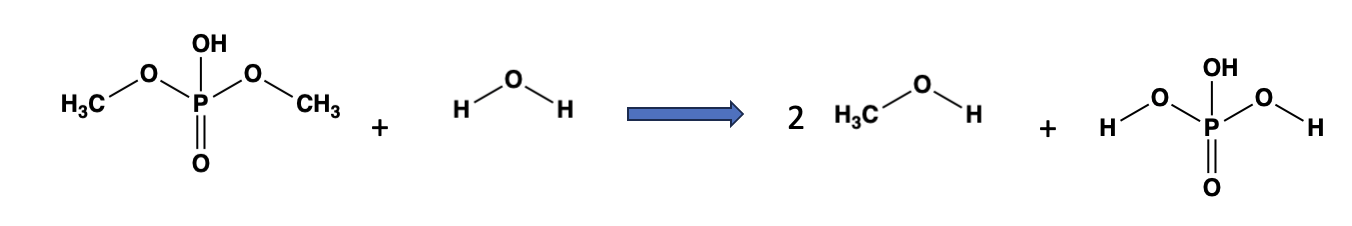
Figure 2. Hydrolysis of phosphate esters is conceptually identical to that of "traditional" esters. Note that instead of a carboxylic acid, one molecule of phosphoric acid and two molecules of methanol are formed.
What does any of this have to do with the instability of RNA? Time to roll out the chemistry.
It's All About Ring Size
Without getting into the details of organic chemistry, it’s useful to know that compounds, natural or synthetic, can exist in a linear (straight-chain) form, while others exist in a cyclic (ring) form. Linear molecules are flexible chains of atoms, whereas cyclic molecules are built around closed rings. Both types are common in nature, each suited to different biological roles (Figure 3).
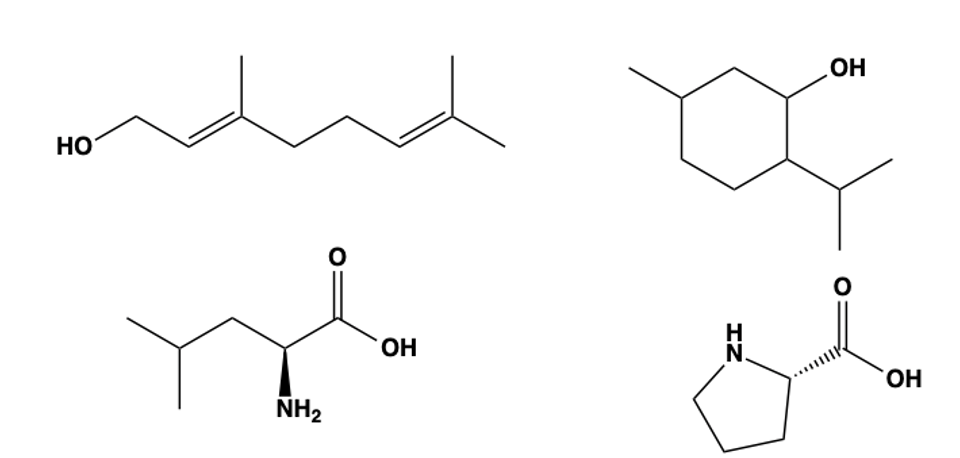
Figure 3. (Top) Geraniol (acyclic) and menthol (cyclic), two common natural scents. (Bottom) Leucine (acyclic) and proline (cyclic) are both amino acids.
Messenger RNA Instability
This may sound obscure as it relates to COVID vaccines, but it is anything but. The propensity of acyclic molecules to form cyclic versions depends significantly upon the size of the ring that forms.
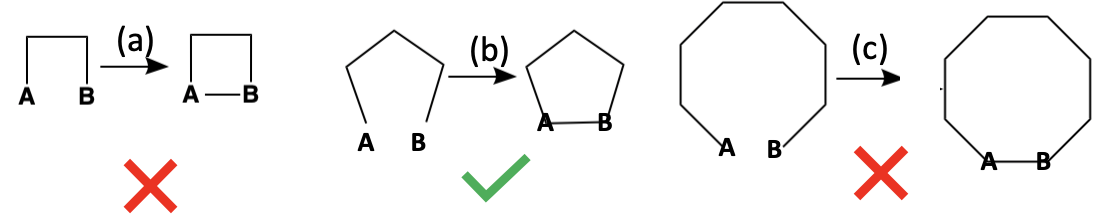
Figure 4. The ease of ring formation depends on size.
Open chains of three or four carbons (Left) rarely cyclize because their ends can only meet by forcing the atoms into highly strained angles, resulting in rings with severe ring strain. Chains of seven or more carbons (Right) are so flexible that their ends seldom align properly, making ring closure entropically unfavorable. By contrast, five- and six-carbon chains (Center) have just the right length and geometry for their ends to meet, form bonds with minimal ring strain, and react efficiently — which is why the vast majority of natural products contain rings of this size.
What does this have to do with the instability of RNA?
A lot. In Figure 5, we see a segment of RNA (Left). Note that a hydroxyl group (called 2'-hydroxy) is five atoms (double arrow) from the phosphorous atom of the phosphate ester. This makes RNA ideally suited to form a 5-membered cyclic phosphate (Right, red oval). Once this happens, the phosphate bond (red hatched line) breaks and the remaining RNA (blue oval) falls apart. In other words, RNA is built to break itself down.
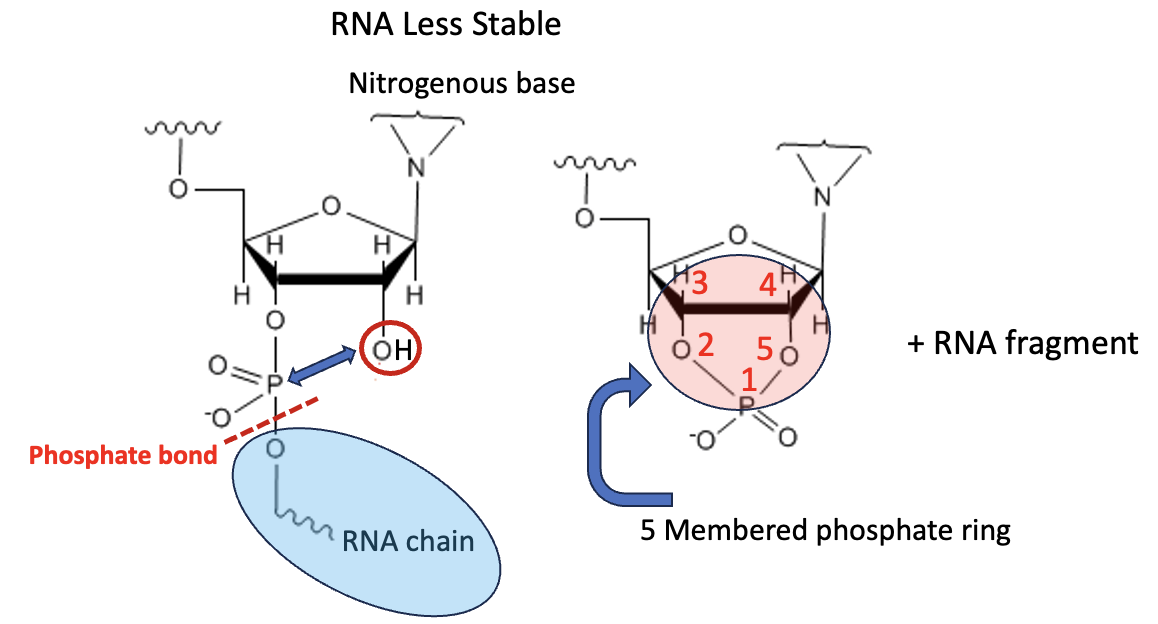
Figure 5. Autocleavage of RNA.
By contrast, DNA contains a non-reactive hydrogen atom (green circle) instead of the hydroxyl group in the 2' position (Figure 6). While this may seem like a small change in a huge molecule, the opposite is true. In the absence of the 2'-hydroxyl group, the same phosphate bond is exceptionally stable.
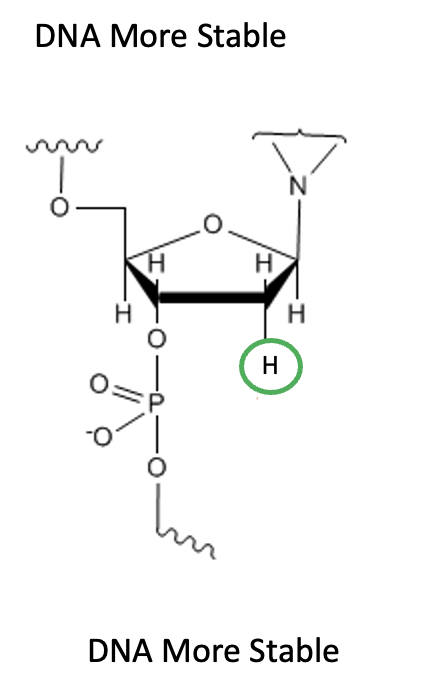
Figure 6. DNA, which lacks the 2'-hydroxyl group of RNA, is exceptionally stable.
Bottom Line (Science Only)
So, it really just comes down to this:
DNA differs from RNA by one oxygen atom, a seemingly trivial change in a huge molecule. But that's all it takes to make RNA less stable than DNA. While RNA decomposes in minutes or hours, DNA can survive for centuries under favorable conditions.
People may argue about the safety of mRNA vaccines, but there is no argument about the difference between RNA and DNA. They have evolved for specific purposes [1], which are governed by the underlying organic chemistry – a science that has long been established and is independent of political considerations.
NOTE:
[1] I have intentionally omitted the enzymatic cleavage of RNA, which is much faster than the chemical reaction. The reason? The enzymatic reaction is identical to the chemical reaction, both occurring via the cyclic phosphate ester shown in Figure 6. The enzymes (RNases) simply make it faster.



