
While drug shortages are neither new nor uncommon, I must admit I was surprised to read about the current aspirin shortage in the UK. It's gotten so bad that some pharmacies are rationing it, while many others have none. This brings up an important question.
Let's say that a British heart patient who can't locate any aspirin finds a two-year-old bottle in the medicine cabinet. When the bottle is opened, there's a faint vinegar smell. Surely this bottle is "spoiled" and needs to be tossed, right?
Wrong.
The presence of acetic acid in a bottle of aspirin is perfectly normal. Maybe even likely. This can easily be explained by simple chemistry (assuming there is such a thing).
325 mg Worth of Aspirin Chemistry
Aspirin (acetylsalicylic acid, ASA) belongs to a class of organic molecules called esters. Esters are formed when alcohols react with carboxylic acids; a molecule of water is lost in the process. Here's a simple example (Figure 1).
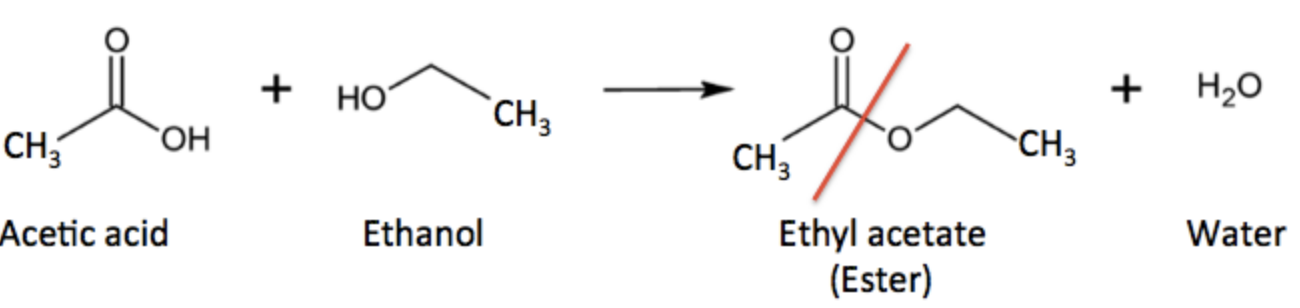
Figure 1. Acetic acid (a carboxylic acid) reacts with ethanol (an alcohol) to form ethyl acetate (an ester) and water. The red line shows the new ester bond.
But ester formation is reversible; esters react slowly [1] with water to give back the same alcohol and carboxylic acid that they were formed from. This is an example of a hydrolysis reaction; water reacts with a substrate (usually an ester or amide) to break it into two fragments. (Figure 2).
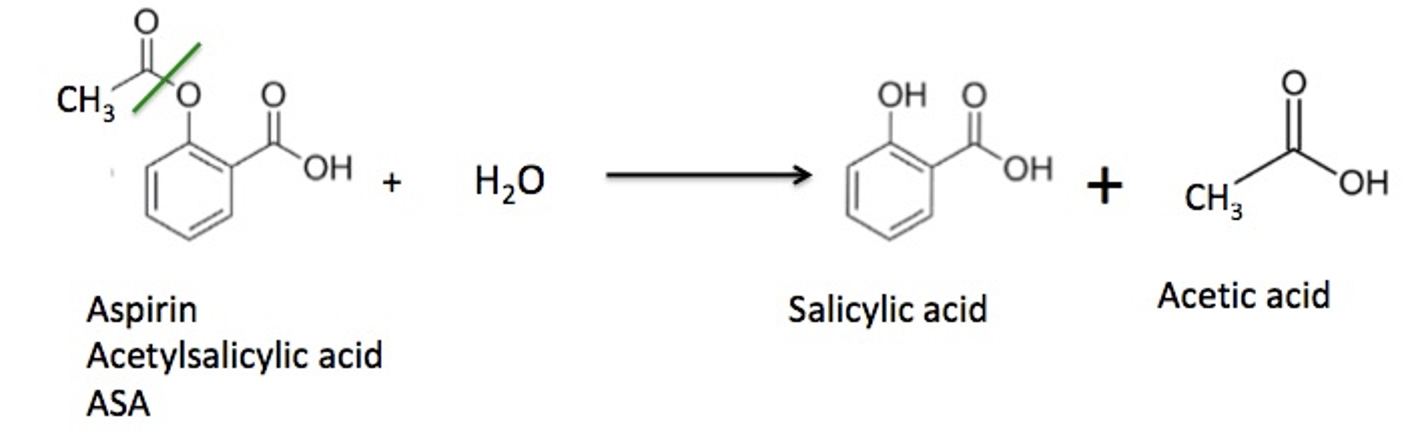
Figure 2. The reaction of aspirin with water to form salicylic acid and acetic acid. The green line shows the ester bond that is broken during the hydrolysis reaction.
Smell Can't Tell
Let’s do some math. A bottle of 100 pills at 325 mg each contains 32.5 grams of aspirin. If that aspirin is 99.99% pure, the remaining 0.01% corresponds to roughly 3 milligrams of acetic acid, produced by aspirin hydrolysis. That works out to about 100 parts per million (ppm) by weight.
Humans, however, can detect the smell of acetic acid at concentrations of around 0.03 ppm in air. Even though these numbers describe different things—the amount present in a bottle versus the detection limit in air [2]—the contrast is striking. Very small amounts of aspirin hydrolysis can generate orders of magnitude more acetic acid than is needed to produce a noticeable vinegar odor, which is why smell is only a “yes or no” signal that some hydrolysis has occurred, not a reliable measure of how much aspirin has actually lost its potency.
So, when you open an old bottle of aspirin, it is common to smell vinegar. This means that at least some of the aspirin has hydrolyzed. But does it matter? Very unlikely. Therefore, odor is a “yes/no” indicator of some hydrolysis, not a quantitative assay of potency loss.
Ye Olde Pharmacy
But, suppose the bottle was really old? Would it matter? Not that much.
Vinegar is not only harmless, but red wine vinegar is now advertised as a cure-all for all human ailments. So that isn't going to hurt you. What about the other part of the aspirin molecule - salicylic acid? Will this hurt you? Not really, especially since it is sold right next to aspirin on pharmacy shelves (3).
Doan's Pills contain salicylic acid, magnesium salt.
Salicylic acid is chemically similar to aspirin but not nearly as effective. For some reason, it is sold as an analgesic remedy for back pain. Talk about ridiculous. A very weak version of aspirin is sold specifically for lower back pain, as if your back and not the rest of you knows that it's supposed to work. The only reason I can imagine why it is still sold is that it has been used forever.

Doan's Pills ca. 1950. Photo: Creator: Ellen Castron/ National Museum of American History, Smithsonian, Open access.
Bottom line
If you want to throw out old aspirin, fine with me. Just know that its degradation products are both touted as health remedies.
NOTES:
[1] This reaction is greatly accelerated by the presence of either acid or base.



