 There is an op-ed in today s New York Times written by former Obama administration health guru Dr. Ezekiel Emanuel, which supposedly addresses the dire need for new antibiotics which is both wrong and misleading. Not only is the piece deeply flawed, but Emanuel s proposed solution to address lack of new antibiotics is not only infeasible as stated, but is already being implemented in a different form.
There is an op-ed in today s New York Times written by former Obama administration health guru Dr. Ezekiel Emanuel, which supposedly addresses the dire need for new antibiotics which is both wrong and misleading. Not only is the piece deeply flawed, but Emanuel s proposed solution to address lack of new antibiotics is not only infeasible as stated, but is already being implemented in a different form.
ACSH s Dr. Josh Bloom says, The introduction is fine, but once the facts start to appear, it gets dicey fast. Most obvious is Emanuel s bias against the pharmaceutical industry. If one were to take his points at face value, one could only conclude that the problems we now face are simply a matter of greed: Drug companies abandoned antibiotic research simply to move into more profitable areas.
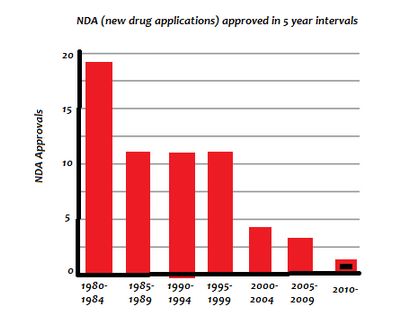
Emanuel writes, The number of F.D.A.-approved antibiotics has decreased steadily in the past two decades. The big pharmaceutical companies have largely stopped work on these drugs. Also, The big problem is profitability.
Dr. Bloom says, Either intentionally or because of a poor understanding of the subject, Emanuel s analysis leads readers to certain conclusions. But his omission of certain facts paints an inaccurate picture of what really happened: The FDA screwed up big time two decades ago. This is a big part of why we are in the mess we now find ourselves in, something that Emanuel fails to even mention.
When in doubt about anything regarding antibiotics, the person to turn to is ACSH advisor Dr. David Shlaes, the former vice president of infectious disease research at Wyeth, and one of the premier experts on all aspects of the topic, from the science to politics of regulation. Shlaes blog, Antibiotics-The Perfect Storm, is the place to go to get the facts straight. (Shlaes has also written a book with the same title).
Drug companies did not simply drop out of antibiotic research they were forced out.
Shlaes explained why this happened in a 2002 letter that was published in the Journal Clinical Infectious Diseases, where he criticised a fundamental change in FDA policy that led to drug companies dropping their antibiotic programs.
He and co-author Robert Moellering wrote, This requirement [new and unrealistic parameters for clinical trials of new antibiotics] which seems innocent and technical, threw the pharmaceutical industry into a panic and probably contributed to the recent withdrawals by Lilly and Bristol-Myers Squibb from the antibacterial discovery business.
Shlaes and Moellering were prescient:
Dr. Bloom says, This is the main reason why antibiotic research was abandoned: a ridiculous change in the FDA s policy. Yet, it would take until 2012 for the FDA to even acknowledge that they had to reboot their policy, and another two years before it actually would begin to happen.
(We suggest that you read Shlaes Science 2.0 piece on how the U.S. lags behind Europe in antibiotic development).
What does Shlaes think about Emanuel s idea? Apparently not much: The New York Times is my favorite newspaper. I devour it every day. I believe that it is the best in the world (some Londoners might argue this). So I was both overjoyed and shocked to see an editorial in the Times today by Ezekiel J. Emanuel who argues that we need to offer prizes for antibiotics active against resistant pathogens.
Also, Although this might be a good idea it s not new and certainly does not belong to Dr. Emanuel. It's been a topic of high-level talks for 6 years.
Dr. Bloom, who has published extensively on this topic, including pieces in The Wall Street Journal, National Review Online, and The New York Post, doesn t think that Emanuel s solution is feasible.
Emanuel writes, What if the United States government maybe in cooperation with the European Union and Japan offered a $2 billion prize to the first five companies or academic centers that develop and get regulatory approval for a new class of antibiotics? And Because it costs at least $1 billion to develop a new drug, the prize money could provide a 100 percent return.
To which Dr. Bloom replies, First of all, $1 billion is really $2.5 billion. But Emanuel s reward system reveals a fundamental lack of understanding of the chemistry and biology behind antibiotic discovery. A class is a rather subjective term. It can be interpreted in a number of different ways. Two different chemists can look at two different chemical structures and have a very different idea of whether they are in the same or different classes. And, if you define class by antibacterial properties, it gets even murkier. With this much money at stake this will become an issue that will probably end up in court. Just what we don t need.

