Multiple myeloma is a type of blood cancer. B-cells, immune cells that play a crucial role in adaptive immunity, differentiate into plasma cells that secrete the antibodies we need to fight infections and other foreign invaders. Multiple myeloma is a cancer of these plasma cells.
A common treatment for multiple myeloma is itself an antibody called daratumumab. (Pro tip: A drug name that contains the suffix -mab indicates the drug is an antibody.) The antibody targets a cell membrane protein called CD38, which is highly expressed on malignant plasma cells. This triggers an immune response against the malignant cells, resulting in their death.
B-cells and plasma cells also play a role in allergies. Allergies result from an improper adaptive immune response that produces antibodies against harmless substances, like pollen and peanuts. (See diagram.)
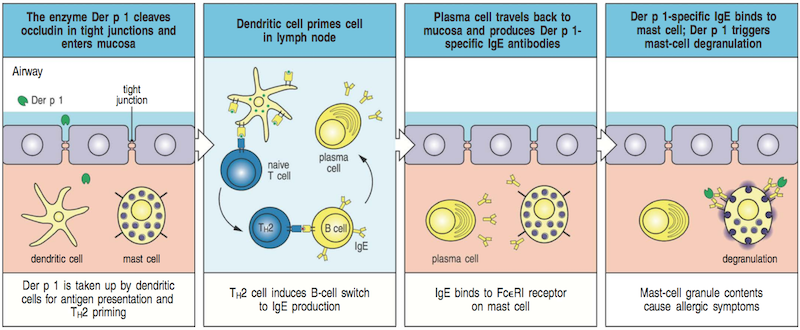
Credit: Janeway Immunobiology, 8th ed.
In short, a type of antibody called IgE is produced in response to an allergen, after which it binds to the surface of mast cells, another type of immune cell. When a person is exposed again to the same harmless substance, the IgE antibodies bind to it and cause the mast cells on which they are attached to go crazy ("degranulate"), releasing substances that cause all the symptoms associated with allergies.
The common link, therefore, between multiple myeloma and severe allergies is the plasma cell. In the former, plasma cells are cancerous, meaning there are too many of them; in the latter, plasma cells produce harmful IgE antibodies. If daratumumab can treat multiple myeloma by killing off plasma cells, then it's possible it could also treat severe allergies.
So a team of researchers based mostly in the Netherlands tested that hypothesis with an observational study of eight patients with multiple myeloma who had received daratumumab therapy. (Some patients were receiving other drugs, as well.) They monitored the level of IgE present in their blood at baseline and after taking daratumumab.
At baseline, only one of the patients had IgE levels that were considered abnormally high. He also tested positive for allergies to common substances, such as grass pollen and house dust mite. Treatment with daratumumab caused a marked decline in the patient's IgE levels.
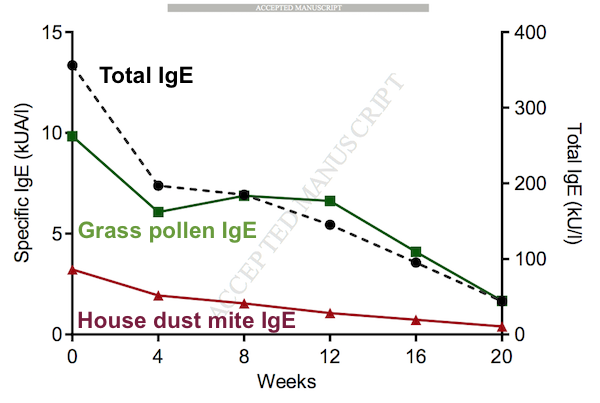
Additionally, three other patients with normal levels of IgE also experienced declines following treatment with daratumumab. (The other four patients did not have detectable IgE levels at baseline.)
The authors did not examine whether the patient actually experienced fewer allergies. This study serves only as a proof-of-concept that provides a scientific justification for further examination. Since daratumumab is already approved for multiple myeloma, it should be relatively easy to begin clinical trials aimed at treating severe allergies.
Source: Blankestijn MA, van de Donk NWCJ, Sasser K, Knulst AC, Otten HG. "Could daratumumab be used to treat severe allergy?" Journal of Allergy and Clinical Immunology. Published: 19-Jan-2017. doi: 10.1016/j.jaci.2016.12.955.