Inside of our body cells are tiny little organs ("organelles") called mitochondria that serve as miniature power plants. They provide the energy necessary for metabolism to occur and our bodies to function.
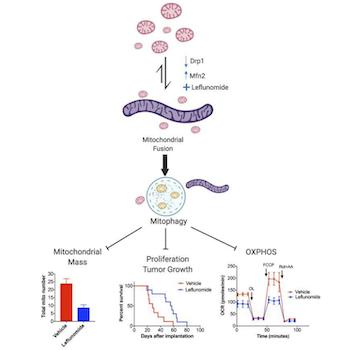
Mitochondria are not static structures. They can bend and move and even divide or fuse with other mitochondria. Collectively known as "mitochondrial dynamics," mitochondrial fission and fusion play a role in a cell's response to stress. As might be expected, mitochondrial dynamics can go awry inside cancer cells.
The most common type of pancreatic cancer is PDAC, pancreatic ductal adenocarcinoma, which is a tumor that forms in the ducts (tubes) that deliver pancreatic juices (that aid in digestion) to the small intestine. The mitochondria of PDAC cells are unusually fragmented, indicating that processes promoting mitochondrial fission (division) outpace those that promote fusion.
Now, scientists believe that this imbalance in mitochondrial dynamics could serve as a target in the treatment of pancreatic cancer. Using a mouse model, researchers describe in the journal JCI Insights that an FDA-approved drug for the treatment of arthritis also happens to promote mitochondrial fusion in pancreatic cancer cells and, crucially, extends survival time.
 How an Arthritis Drug Could Help Treat Pancreatic Cancer
How an Arthritis Drug Could Help Treat Pancreatic Cancer
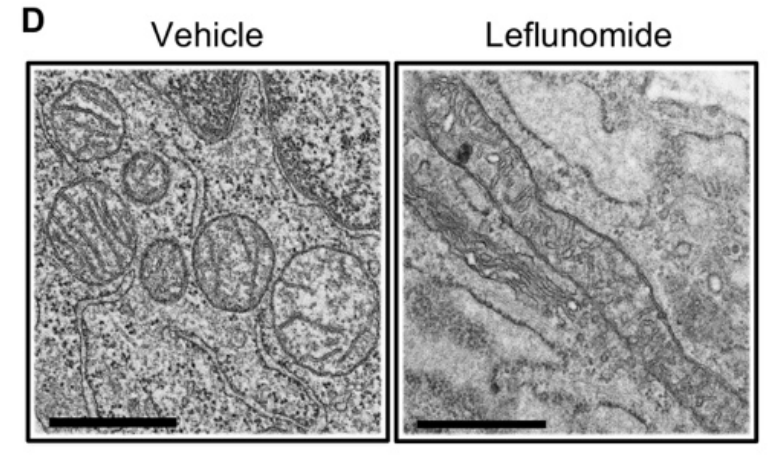
How does the drug, called leflunomide, work? Based on their investigation, the authors showed that it increased the expression of a protein that is involved in mitochondrial fusion. A beautiful micrograph (see figure) shows the striking changes that occur inside cancer cells exposed to the drug. The left side of the figure depicts a control with fragmented mitochondria; the right side depicts a long, fused, tubular mitochondrion in response to leflunomide treatment.
The cancer cells don't seem to be very happy with this. The authors then demonstrated that the fused mitochondria are systematically destroyed in a process called mitophagy. From the cancer cell's perspective, this is counterproductive because it is destroying its own power supply. As a result, in various mouse models of pancreatic cancer, leflunomide prolonged survival.
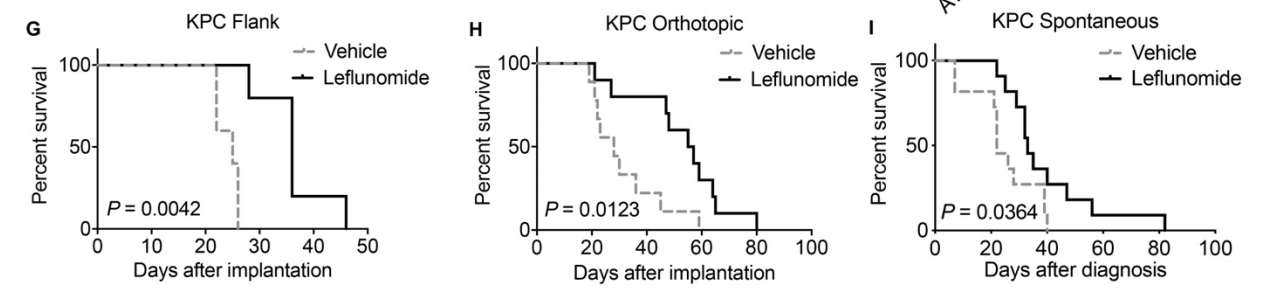
All the usual caveats applicable to animal studies apply to this research, as well. What's interesting, however, is that this paper serves as a sort of proof-of-principle demonstrating that dysfunctional mitochondrial dynamics could be a viable target in cancer therapy. And because leflunomide is already FDA-approved, it should be relatively easy to get it into clinical trials for pancreatic cancer.
Source: Meifang Yu, et al. "Mitochondrial fusion exploits a therapeutic vulnerability of pancreatic cancer." JCI Insight. In press. Published online: 23-July-2019. DOI: 10.1172/jci.insight.126915