There is now a potentially important change in the pharmaceutical landscape of anti-coronavirus drugs. Favipiravir, a little-used six-year-old drug from Japan probably isn't a game-changer, at least not yet, but given the urgency of having a drug that can at least control coronavirus infection, favipiravir joins Gilead's remdesivir in the spotlight. (See Can Gilead's Remdesivir Tame The Coronavirus?).
Before we get into this I may need to defend myself, so let's get that out of the way. Following are quotes from some of my recent articles:
"You have to be out of your gourd to put money on a drug based on Phase II data." 3/2/20
"Given the properties of remdesivir as well as the desperate need for a coronavirus therapy, I would estimate that the drug has at least a 50% chance of becoming the first drug to treat the virus." 3/16/20
"Favipiravir is unlikely to be a successful coronavirus drug." 3/16/20
The first statement is like saying water is wet. The other two are based on these data:
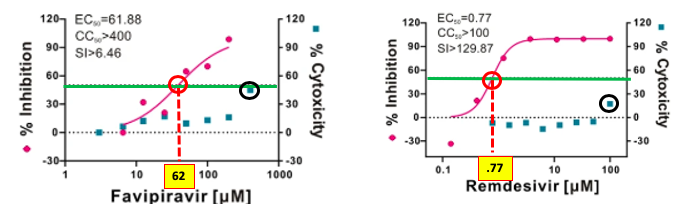
Dose-response curves of favipiravir (Left) and remdesivir (Right). The red hatch lines indicate the concentration of the drug required to inhibit 50% of viral growth (this is called an EC50). The lower the EC50 the more potent the drug. Favipiravir is a weak inhibitor of coronavirus replication (62 micromolar - a very high number for a drug). Remdesivir is 80-times more potent (EC50 = 0.77 micromolar), putting it in the same range of other antiviral drugs. Source: Nature
All things equal, remdesivir should be a better drug than favipiravir, since it is 80-times more potent, but in drug discovery, all things are never equal. Potency is only one of the dozens of parameters that determine whether a drug will be successful or not.
Long-time chemistry blogger Derek Lowe had the same take in his March 6th blog.
Unfortunately, from what I can see, [favipiravir] has already turned up as ineffective in in vitro tests. The human trial that’s underway is honestly the sort of thing that would only happen under circumstances like the present: a developing epidemic with a new pathogen and no real standard of care. I hold out little hope for this one.
MEA CULPA WITH A DISCLAIMER
So, maybe I was wrong in the 3/16 article – it wouldn't be the first time. This remains to be seen but now, at least upon first glance, it looks like favipiravir is performing better than I (or Derek) would have expected. Here's the new information.
FAVIPIRAVIR ENTERS THE FRAY
Favipiravir (brand name Avigan) is an existing drug that was developed by Fujifilm Toyama Chemical and approved in Japan in 2014 for the treatment of influenza. It has been undergoing clinical trials in China and been used therapeutically in people who are infected in Japan. The drug is not approved in the US; it doesn't work very well for flu, but in 2014 when Fujifilm announced the approval of the drug there was one very important paragraph (emphasis mine):
AVIGAN is a viral RNA polymerase inhibitor with a new mechanism of action, inhibiting viral gene replication within infected cells to prevent the propagation. Due to this characteristic, the drug is expected to have an antiviral effect on Avian Influenza A (H5N1 and H7N9) and other viruses, with efficacy already confirmed in animal studies.
The key is "other viruses." Avigan has been used with limited success against other RNA viruses, such as Ebola, West Nile, and Zika.
How well does it work against coronavirus? The results are mixed:
- In one clinical trial of 200 patients in China, people who received the drug cleared the virus (negative test) in four days vs. 11 days for those did not.
- In another trial, patients treated with favipiravir recovered from fever in 2.5 days vs. 4.2 days and also stopped coughing within 4.6 days vs. patients who did not take the drug.
- (This one is big) Patients who took the drug needed to be put on a ventilator half as often as those who didn't.
CHINA SAID, JAPAN SAID
Perhaps the most intriguing aspect of this story is that very different information is coming from the two countries.
"The drug is very safe and clearly effective...there were no clear side effects."
Zhang Xinmin, director of the Chinese government’s National Center for Biotechnology Development
The story in Japan is quite different. Favipiravir, like all drugs, comes with its own set of baggage and favipiravir has a couple of extra carry-on bags. One of the conditions of approval in Japan was that it would only be allowed to treat serious infectious diseases like avian flu or Ebola for which there were no viable options. This is because the drug is known to cause birth defects and fetal death.
And one Japanese health official reached a decidedly different conclusion about the efficacy of the drug.
"We’ve given Avigan to 70 to 80 people, but it doesn’t seem to work that well when the virus has already multiplied."
Unnamed source from the Japanese Health Ministry, reported in Mainichi Shimbun (one of the major newspapers in Japan.
FAVIPIRAVIR SUMMARY
Some of the data from trials in China seem impressive but will need to be reproducible in a larger group of people and in a different country. Furthermore, to be the opinions of Japanese scientists are sobering. It is impossible to know which country "wins." On balance, I would say the news is positive, maybe even very positive, especially if the efficacy data holds up in further trials. But be prepared for surprises. The only thing that is clear here is nothing.



