Small particles less than 0.3 microns may be harboring the SARS-COVID-2 virus and may represent a surrogate monitor of SARS-COVID-2 infection
An air monitoring instrument that measures viable SARS-COVID-2 particles in our exhaled breath does not yet exist. In its absence, surrogate measures of virus numbers and estimates of potential infectivity from room air exposures have been reported – the presence of particles in the size range of viruses or the droplets of moisture that they travel upon. We made use of such an approved device that measures “fine dust” within the environment, quantifying amounts categorized by size. The device, the Resp-Aer-Meter, heats the flowing air sample to 70 degrees C. to prevent condensation of humid air followed by counting and sizing of particles.
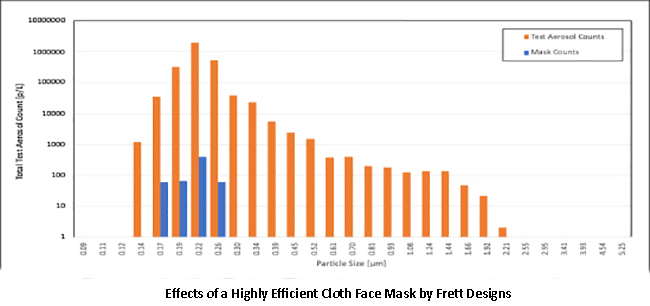
We tested a currently available, reusable, washable cloth reusable mask, rated [1] to be equivalent by our measure to the widely available single-use or disposable N95 masks, using this instrument. The mask, by Frett Design of Canada, was tested by exposure to aerosols of 300 nm silica beads in the air (to simulate viral aerosols) - a cloud that was custom generated from a 0.5% suspension of beads in highly purified distilled and deionized water. This example shows a near theoretical 99.9% filtration efficiency.
The airborne nature of respiratory viruses like COVID-19 is well recognized. Based on work showing that the nasal sinus harbors the SARS-COVID-2 infection, we hypothesized that elevated nasal concentrations of the virus might constitute a second human source of infectivity after the lung emissions via the mouth. With the availability of a purpose-built instrument, we undertook, on a volunteer basis, to monitor both oropharyngeal (mouth) and nasal sinus emissions in our healthy workgroup.
This graphic shows the mouth and nose surveillance results by particle size in our cohort.
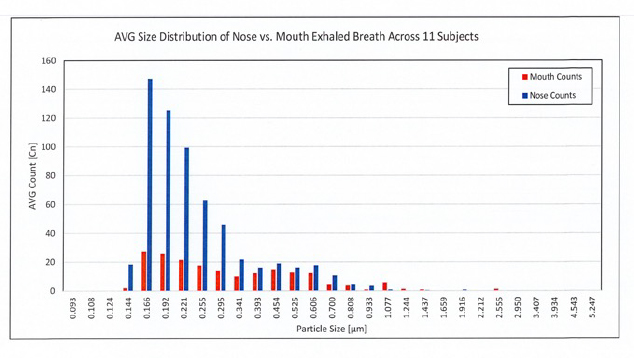
The magnitude of the difference between the two respiratory outputs is substantial.
The predominant particle size is less than 2 microns, and the concentration of nasal emissions, based on size, were up to six-fold greater than those from the mouth. Other investigators have reported larger size values most often associated with singing, talking, sneezing, or coughing.
We hypothesized that the respiratory emissions from the nose might show an earlier or altered pattern of infective aerosol release in the expired air of persons ill with SARS-COVID-2.
One of our voluntary test group, fully vaccinated and boosted, developed COVID-like symptoms after exposure to a work colleague who developed COVID-19. For a variety of reasons, they were able to test their nasal and oral exhalations without endangering other employees.
Here is a portion of the results of that series of observations for the size distribution of nasal sinus emissions.
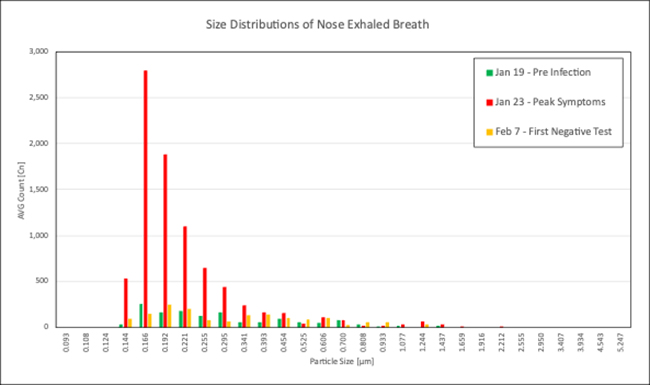
There are two significant findings.
- There is a profound ten-fold increase in particulates from the nasal outlet in the presence of symptoms, consistent with the airborne transmission of the virus.
- The second is the almost exclusive presence of particles less than 0.3 microns identified by this measurement method.
While this is a case report (study with an N of 1), these findings support the use of nasal emissions in this particle size range (< 0.3 nm) as a means of surveillance for a potentially infectious person, whether symptomatic or not. This single observation obviously requires replication on a larger scale by others. A new report in MedRxiv, involving 500 adults is consistent with our observations. They showed elevated oropharyngeal particle concentrations, less than 0.3-micron size in COVID-positive patients, and observed this in large excess emissions above that seen in healthy subjects.
[1] under American Society for Testing and Materials F3502 guidelines
I would like to thank Dritan Xhilari, M.S Che., a member of our team, who did the plotting, tabulation, and statistics for this article.