Sometimes you just run across a study or paper that is really clever. Although I've never given much (make that any) thought to the importance of drug palatability as it pertains to compliance, this is where the clever part comes in. Alkaloids – nitrogen-containing chemicals derived from plants – are notoriously bitter, so much so that children won't swallow certain bitter drugs, making compliance a problem.
This is the basis of some nifty work by Chunju Li and colleagues at the College of Chemistry, Tianjin Normal University in Tianjin, China, which appeared in a recent paper in the journal Chemical Communications.
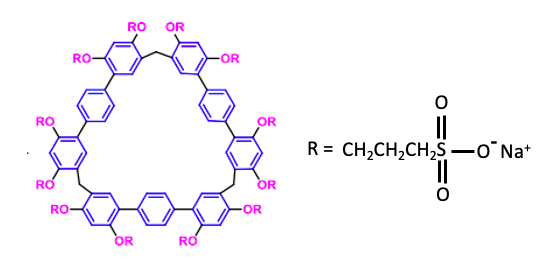
Figure 1. The chemical structure of a sulphonatopropoxy terphen[3]arene, mercifully nicknamed STP3. The nine interconnected benzene rings form a macrocyclic (1) host that is the correct size for the guests – bitter drugs – to fit inside. The 12 ionic sulfonate groups (OR in pink) make the complex water-soluble and also non-toxic (2). Source: Chunju Li et. al., Chem. Commun., 2022, Advance Article, DOI: https://doi.org/10.1039/D2CC00040G. This paper is the source of all figures and tables used below.
The group demonstrated the formation of the host-guest complex by fluorescence spectrometry. It is clear from the read-out curves that a 1:1 complex does form. This should not be surprising given the fit of the drug inside the host (Figure 2).
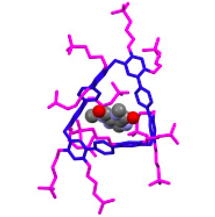
Figure 2. The structure of a host-guest complex between STP3 and theophylline. Snug as a bug in a rug.
The group tested its host-guest bitterness against the guest (drug) alone. The ability of the host to mask the drug was determined by comparing the bitterness of the drug vs. that of the complex. How does one do this? By use of an electronic tongue, of course. (Note to perverts – comments regarding this technique are unwelcome. More or less.)
Here are the structures of the bitter alkaloid drugs and their utility as drugs (Figure 3).
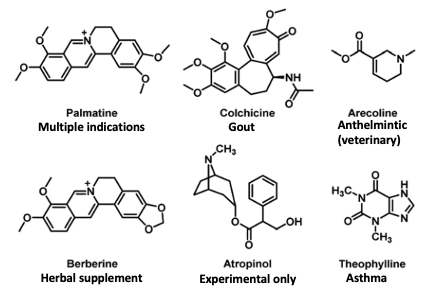
Figure 3. The structures and medical use (if any) of the six alkaloids tested for bitterness.
Did it work?
Some of the time. Below is a modified table that compares the bitterness of the six drugs and their complexes. The higher the number, the more bitter the drug is.
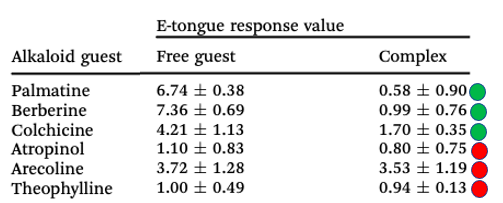
Table 1. A comparison of the bitterness of six drugs with that of their ST3 complexes. The green circles indicate a positive result. The red circles indicate little or no effect.
What's going on?
Although all six drugs form 1:1 complexes with STP3, a reduction in bitterness is only apparent in half of them. The authors postulate (and this makes sense intuitively) that the degree reduction of bitterness in the complex is a function of the bitterness of the drug itself. In other words, if the drug is very bitter, nesting it within the host makes it much less so. If the drug isn't especially bitter, then the effect is less/none, suggesting that there is a lower limit of bitterness somewhere around 1.0. In Table 1, the three most bitter drugs get the green circles, and the three least bitter get the red circles. (These results were confirmed in mouse two-bottle drinking experiments.)
Chemistry is cool
Here is a simple but elegant solution applied to a real-world problem and helps solve that problem. A bitter drug is designed to fit into a "protective cage," which masks an undesirable property – in this case, taste. Then, upon ingestion, the drug separates from the host and does its job while the host goes on its merry way leaving behind the medicine to do its job.
Sweet.
NOTE:
(1) A macrocycle is simply a chemical containing a ring with many atoms.
(2) Highly polar molecules, especially large ones, are generally not absorbed in the gut. They are typically excreted unchanged in the urine. STP3 was also shown to be non-toxic in several standard toxicity assays. The ionic sulfonate groups make the molecule polar and water-soluble.




