There's considerable ongoing research to develop analgesic medications that don't have the liabilities of NSAIDs and opioids. Many potential drugs are in various stages of development. Most will fail. This multi-part series will examine drugs in development and their potential utility. First up is VX-458, a Phase III candidate from Vertex.
Does the term "non-opioid analgesics" make you automatically cringe? It should because when the anti-opioid zealots use this term we all know that current options (and I use that word loosely) include drugs and "therapies" that range from useless to quite possibly dangerous. Some of these include:
- Acetaminophen (Tylenol) – Virtually useless and a potent liver toxin.
- NSAIDs (e.g., ibuprofen, aspirin) – Effective for many types of pain but can be murder on the stomach and kidneys and also cause bleeding, heart attacks, and stroke, especially in elderly patients. Some people cannot take them at all.
- Antidepressants – Virtually useless.
- Neurontin, pregabalin – Some utility for neurological pain. Multiple side effects.
- Yoga, mindfulness, meditation, music. Especially helpful for people who have no pain.
Fortunately, the pharmaceutical industry is working to tackle the enormous unmet medical need for safer and more effective analgesic drugs. But, historically at least, the odds will always be against them; both heroin (opioid) and aspirin (NSAID) were discovered in the 1890s. Yet 130 years later opioids and NSAIDs are still the go-to drugs for pain. It would not be unreasonable to say that research to discover novel drugs to safely and effectively treat pain is the most challenging of all diseases and conditions. After well more than a century of research, there is little to show.
However, this hasn't stopped numerous companies from wading into the area. According to Digital Journal, there are more than 55 non-opioid drugs in development for postoperative pain (1). Many (most?) of these will fail. Some have already failed, as pointed out by Elizabeth Cairns, who wrote "Drug makers leave the pain behind" for the Evaluate Vantage site in 2022. Cairns has lists of drugs that have been dropped and those that are hanging on (some for dear life). Note: The Evaluate Vantage article covers both drugs for post-op and also chronic pain, something many readers will focus on.
Following is the beginning of a series focused on the clinical status of novel analgesic drugs in development, including ups and downs, the stage of clinical development, data from ongoing trials, and planned trials, if any.
VX-548 (Vertex)

The chemical structure of VX-548
It's no accident that I'm starting with Vertex, a Boston-based biotech (2), one of the premier companies in drug discovery research. In 2020 Fortune Magazine rated the company #3 in innovation.
The company's experimental pain medication, VX-548, is (at the very least) interesting, perhaps more.
Following two positive Phase II clinical trials, the FDA gave the company breakthrough therapy designation for acute pain, meaning that its Phase III trials (already begun) will go to the head of the line.
Below is a summary of the Phase II trials, where the pain relief of the drug for bunion surgery and abdominoplasty (tummy tuck), both notoriously painful surgeries, was evaluated.
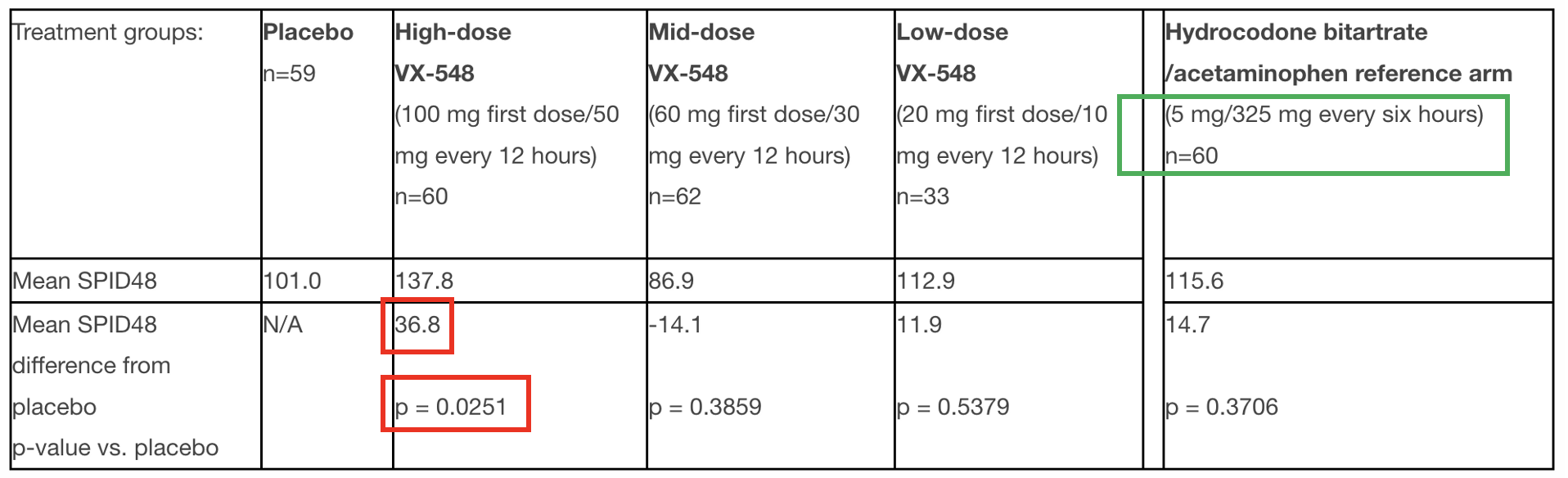
Pain reduction following bunionectomy. Shown are the Sum of Pain Intensity Difference over 48 hours (SPID48), which measures the relief from placebo (left) VX-548 (three different doses), and Vicodin (right). Source: BusinessWire
The key parameters are the difference in pain between placebo, VX-458, and hydrocodone/acetaminophen (Vicodin 5/325). Note that VX-548 affects pain scores significantly and statically (red boxes) at the high dose only. The mid-dose and low-dose arms showed no efficacy. I wonder about the positive control of Vicodin 5/325. Vicodin is the weakest of the commonly used opioids, and 5 mg is the lowest therapeutic dose. It would have been interesting to see the Vertex drug compared to Percocet 10/325, a higher dose of a stronger opioid. Yet, a drug that performs better than a therapeutic dose of Vicodin is no small accomplishment.
Not shown: Results for abdominoplasty were similar. Side note: I would not want to be in the placebo control group after either operation and wonder about the ethics of including such a group in the trials.
Current status
Phase III trials, "Evaluation of Efficacy and Safety of VX-548 for Acute Pain After an Abdominoplasty" (NCT05558410) with 1,000 participants began in October 2022 and are scheduled to be completed in early 2024.
Best guess
Keep an eye on this one. While I can't tell you exactly what a 37-point difference on the Sum of Pain Intensity Difference over 48 hours (SPID48) scale really means to someone who wakes up from bunion surgery, VX-548 does outperform low-dose Vicodin, which itself is a pretty big deal. Also, it operates by a novel mechanism (selectively blocks a specific sodium channel), and that makes it automatically interesting. Finally, its side effect profile is very good. When you add in an unmet medical need, a very smart company, and the FDA's breakthrough designation, VX-548 becomes an intriguing candidate. All of this guarantees nothing; the odds of approval are still pretty long, but I'll be paying attention to it.
NOTES:
(1) While studies on post-op pain are nice, the need for drugs to control chronic pain is much greater. There is no a priori reason why safe and effective post-op pain medications could not also be useful for chronic pain.
(2) Vertex calls itself a biotech, but this isn't strictly true. The company also does small molecule research based on molecular modeling and medicinal chemistry. This is how the company discovered and developed telaprevir, one of the first direct-acting antiviral drugs that could cure hepatitis C.

