This may sound strange but on some level antiviral drug research is "easy." Make that "easy compared to other therapeutic areas," because those who do research in the antiviral field – something I did for 10 years – have an advantage over scientists who work on other conditions, such as Parkinson's, pancreatic cancer, or autoimmune diseases. This advantage, which is based on pioneering work in the HIV/AIDS field, may lead to the first-ever antiviral drug for respiratory syncytial disease (RSV).
This is no small accomplishment. The search for a drug to treat RSV has been going on for about 50 years (1), but researchers at Enanta Pharmaceuticals may be the first group to the finish line. However, they didn't get this far without help. This help began in 1995 – 14 years after the beginning of the AIDS epidemic in the US – when researchers at Roche introduced saquinavir (Invirase), the first effective direct-acting antiviral drug (DAAD) for the treatment of HIV infection. After the introduction of Invirase, an HIV protease inhibitor, the death rate from AIDS began to drop for the first time. Since that time, drugs have been developed for every step in the life cycle (Figure 1), forming the basis of modern treatment of HIV infection.
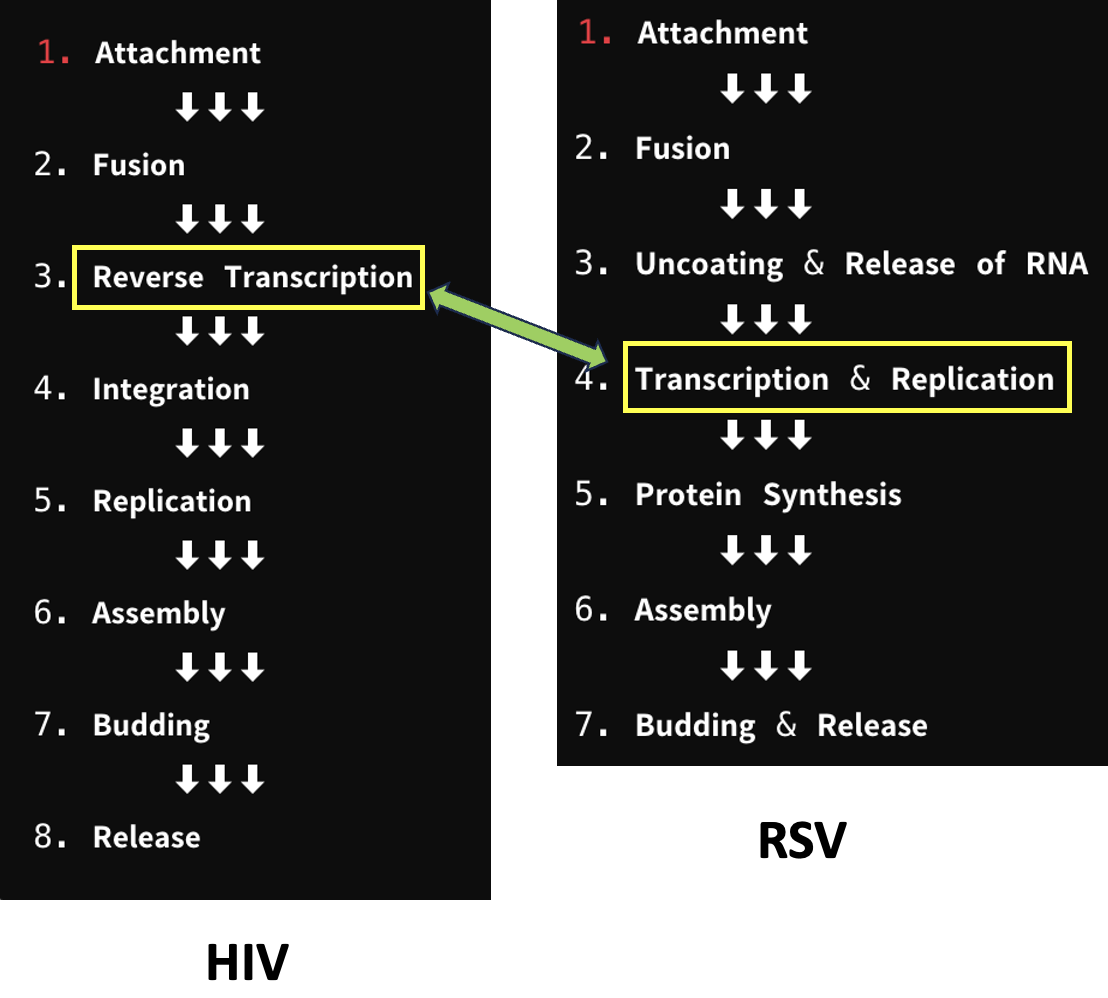
Figure 1. The life cycles of HIV (left) and RSV (right). The life cycle of HIV is similar (but not identical to) that of RSV. As is the case with all viruses, the synthesis of new genetic material (DNA or RNA) is essential for replication. The yellow boxes and green double-headed arrow for both viruses denote this step. The steps -- 8 for HIV and 7 for RSV, are also referred to as targets.
Enanta is developing EDP-323, a drug that inhibits the viral polymerase required to build new genetic material from individual nucleotides. When this step is inhibited, viral replication decreases, resulting in a reduction of viral load. This allows the body to clear the virus more effectively.
A comprehensive reflection on the current state of RSV research is also given, drawing inspiration from the lessons gleaned from HCV and HIV
Felicetti, et. al., J. Med. Chem. Vol 67/Issue 14, July 6, 2024
Why do we need it?
Despite the availability of three new RSV vaccines, a small molecule inhibitor of the virus would represent a crucial advance in treating respiratory infections. RSV causes approximately 2.1 million emergency room visits annually in children under 5, with over 50,000 requiring hospitalization. For adults aged 60 and older, the number of annual hospitalizations ranges from 100,000 to 160,000. The estimated annual cost of treatment in the U.S. alone is $6.6 billion. People remain unvaccinated due to a number of reasons: limited access, medical ineligibility, or vaccine hesitancy. Additionally, children must be at least 8 months old to receive the vaccine, leaving a significant portion of the population unprotected.
What must such a drug do?
To develop a successful antiviral drug a number of criteria must be satisfied:
- The drug must be a potent inhibitor of the virus in cell culture experiments (It must have a low EC50 value, where the EC50 is the concentration that inhibits half of the viral replication.
- The drug must be selective; the TC50 (concentration to kill the cells in culture) must be significantly higher than the EC50 (a high selectivity index).
- The compound must be shown to inhibit the virus by the proposed mechanism.
- The drug must demonstrate favorable pharmacokinetics. In other words, a significant portion of the drug in the pill must get into the blood and remain there long enough to have the desired impact on the virus.
- The concentration of the drug in the blood must exceed that needed to inhibit 90% of viral replication (the EC90).
- The mean blood concentrations of the drug should exceed the EC90 values by significant multiples at the trough (lowest concentration).
- The drug should inhibit all clinically relevant strains of the virus.
- It must do so without severe adverse events.
What did EDP-323 do?
In a Phase 2a human challenge study (2) conducted on 142 healthy adults, once-daily EDP-323 treatment resulted in 85-87% reduction in viral load and 97-98% reduction in infectious viral load, with a 66-78% reduction in symptoms compared to placebo. The drug was found to be safe and well-tolerated. Although these results have not yet been published in a peer-reviewed journal they are impressive by any measure.
- Once-daily oral dosing of a low dose of the drug resulted in blood levels that were 16-fold that of the EC90.
- Once-daily oral dosing of a high dose resulted in blood levels that were 35-fold that of the EC90.
- The drug was dosed for five days and patients followed for another 28 days.
- There were no serious side effects leading to patients withdrawing from the study.
How good are these results?
While working on hepatitis C at Wyeth, we would have killed for results that even resembled these. The potency, bioavailability (3), efficacy, and safety profile of EDP-323 are all covered. Although these data are from Phase 2a trials (and many drugs fail in Phase 3) I see nothing but good news here with the caveat that these results must be repeated in a larger study population. Still, I predict it will do just fine.
Bottom line
Is antiviral research 'easy'? In some respects, yes—thanks to the road map established by AIDS researchers, we have a clearer understanding of how to approach drug development for other viruses. Biologists identify key molecular targets in a virus, and chemists can design molecules to inhibit these targets. When an experimental drug potently inhibits an essential viral target, is non-toxic, and has sufficient bioavailability, it really should prevent the formation of new viral particles within your body. This strategy has led to the development of more than 40 direct-acting antiviral drugs for AIDS and HCV.
As a former researcher, it is especially satisfying to see that Enanta used a strategy that slammed the door on AIDS, cured hepatitis C, and treated COVID-19 can work against a new and important viral target. I wish them well.
NOTES:
(1) Other "treatments" for RSV infections are available, but they are either poorly effective, toxic, or both. Ribavirin has been used, but with little success. Antibodies are available, but these offer passive immunity; they are not therapeutic. The vaccine is not approved for babies younger than 8 months. There is a real need here.
(2) A challenge study is where participants are deliberately infected with a pathogen.
(3) Bioavailability is a measure of the percentage of an oral drug that gets into the blood. The higher the number the better.