Did you drink the Kool-Aid?
No, not the cliché. I mean the real stuff, invented in the 1920s. If so, and grape was your flavor – it was the only one you could plausibly gag down – then you’ve already met the star of this story. Now you can drink it again and swallow this article.
There’s a simple chemical that’s both natural and artificial, scares away birds, and even makes your urine smell “grapey.” It shows up in some grapes but not others. While it isn’t itself an artificial sweetener, it was once used to make the very first one (more on that later). And although it belongs to the aniline family — a group of compounds having an amino group attached to a benzene ring, and often linked with carcinogenicity — this particular one is harmless. Why? See note [1].
When you bite into a Concord grape or sip a glass of grape soda, the distinctive aroma that hits your nose is largely due to a single compound: methyl anthranilate (MA), one of the most recognizable flavor molecules in the food and fragrance industries. Though it may sound exotic, MA occurs naturally in many flowers and fruits, and it has a variety of uses beyond flavoring.
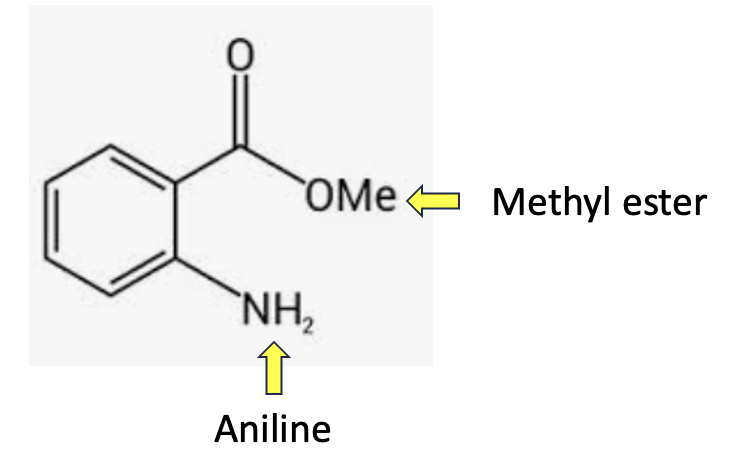
Figure 1. The chemical structure of methyl anthranilate
Natural Origins
Methyl anthranilate is the methyl ester of anthranilic acid, aka 2-aminobenzoic acid – an aromatic amino acid. In nature, it is found in many fruits, such as grapes and oranges, as well as in a variety of flowers, including jasmine and gardenia. As is the case with many fragrance chemicals, it is part of the plant’s volatile "bouquet," helping to attract pollinators with its fruity and floral scent. In Concord grapes, methyl anthranilate reaches concentrations high enough to define the fruit’s characteristic musky aroma that many people associate with grape juice, jams, and candies. But if you're eating Thompson Seedless grapes, there won't be any.
This sensory characteristic is why the flavor and fragrance industries value the compound. Just a small amount can give products a rich, fruity scent. Additionally, MA is used in perfumes to enhance floral blends and in consumer products such as toothpaste and chewing gum. Grape Dubble Bubble Gum! A childhood favorite! One of many things we somehow survived, but is treated like Kryptonite in today’s nutritionally warped world.

Naturally delicious Grape Dubble Bubble, LIK-M-AID, now called Fun Dip, and Kool-Aid
Which, if you remember, always made your pee smell like grapes. Sort of.
You may remember that if you ate or drank grape soda, candy, or Welch's grape juice, your urine may have had a strange, fruity odor. It's methyl anthranilate. But you wanna be chemists out there may be thinking: "Hmm, methyl esters don't hang around very long. They are hydrolyzed in the stomach or in the blood by esterase enzymes. How can this be? (Hint: It has to do with the amino group. Note 2.)
Bird Repellent
Perhaps the most unexpected role of methyl anthranilate is as a bird repellent. It has been approved by the EPA to deter birds such as pigeons and geese. Humans may or may not like it, but birds find it intensely irritating. The compound overstimulates receptors in their trigeminal nerve, producing a burning or painful sensation that leads them to avoid treated areas. This makes methyl anthranilate an appealing alternative to toxic pesticides, since it deters birds without harming them.
Is it safe?
In humans, methyl anthranilate is classified as “Generally Recognized as Safe” (GRAS) by the FDA because of low acute toxicity. In mice, its LD50 is approximately 2900 mg per kilogram body weight, putting it in the range of common substances such as salt, baking soda, vanillin, and MSG. A Concord grape contains about 0.2 mg of MA – information cannot possibly be of any use, no matter the circumstances.
Other (really strange) uses
Time for a pop quiz!
Methyl anthranilate is used in one or more of the following products. Which ones?
a) Eye drops
b) Fireworks
c) Sexual lubricants
d) Artificial sweetener manufacture
e) Transmission fluid
Answers: B and D
b) Fireworks? Why?? It's because it gives the smoke a slightly fruity, sweet smell. There is no one on Earth who would ever guess this; those who may have probably blew up in a July 4th accident.
d) Ironically, although MA is sweet-smelling and not sweet-tasting, it was initially used in the late 1800s to manufacture saccharin, the first artificial sweetener.
A Profoundly Ridiculous Thought Experiment
Consider the following:
- MA is found in (some) sweet grapes. It makes them smell sweet but not taste sweet.
- Likewise, it also makes your urine smell sweet and (presumably) not taste sweet (tell me if you know otherwise)
- It's also used in factories to synthesize saccharin, which tastes sweet but doesn’t smell sweet.
- However, since saccharin is not metabolized in humans, it is excreted unchanged in the urine.
- Ergo, this urine will taste sweet, but not smell sweet; though why anyone might think it wise to verify this theorem remains a mystery.
There must be some reason that I wrote that paragraph. Seemed like a good idea at the time. Perhaps my blood sugar was getting low.
Anyway, if you made it this far, you really did drink the Kool-Aid — twice.
NOTES:
[1] To borrow (and mutilate a phrase from The Graduate: "Vinylogous amide." I shall say no more.






