
It should come as no surprise if you’ve been inundated with health stories about your biome. Do a Google search and—although stupid Google no longer provides result counts—you’d have a better chance of drowning in the Sahara than reaching the end of them.
Hence the disgusting title. Some stomach chemicals [1] are, frankly, unpleasant, and as you’ll see, butyrate—a hot topic in biome research these days—is one of them. Sodium butyrate’s impact on human health ranks high on the list of scientific “Top 40 Hits," including recent articles about its use for liver cancer, general gut health, muscle building in the elderly, obesity, and energy production, to name a few.
In the ever-eccentric world of dietary supplements, there’s usually a kernel of truth hiding somewhere beneath the hype. And without counting the claims du jour, it’s a safe bet that the microbiome—the newest darling of the “explain-everything-that’s-wrong-with-me” crowd—is leading the pack.
So, it’s not surprising that Amazon lists 338 biome products, 120 of which contain the word “butyrate,” with names like Total Few Bio Guard, Gut Intellect, and a book called Biome War Scrolls.
You might want to take care not to mix up your upper-and lowercase letters with this one.

Uh oh. Be careful here.
Let Them Eat Puke — Revolution and Revulsion. And Chemistry.
Whatever this stuff is, it must be important. Before we get into that, a bit of chemistry is required.
Technically, there’s no such thing as plain butyrate — it’s only half a molecule. Butyrate carries a negative charge (an anion) and must be balanced by a positive one, like sodium. Sodium butyrate, the sodium salt of butyric acid (BA), is the actual compound.
Similarly, you can’t buy a jar of “chloride” by itself; sodium chloride or magnesium chloride are real chemicals. "Chloride" is not.
Acid-Base Chemistry and Smell
Ever get a schnoz-ful of hydrogen chloride? If not, this is something you should avoid. It’s an experience you won’t forget. Almost every chemist has had this experience. I have. Not pleasant. It feels a bit like a porcupine crawling up your nose and having a grand mal seizure. Although hydrogen chloride is not technically a tear gas, as in chemical warfare, it is a gas, and it’ll make your eyes water like one. Semantics.
But if you open a bottle of sodium butyrate and take a sniff...well, you're not going to enjoy it. In the bottle is sodium butyrate, the salt form of butyric acid, which is the primary scent associated with vomit.
Butyric acid can also be found in
- Rancid butter (butyrum is Latin for butter)
- Sour milk
- Certain stinky cheeses
- Puke
Why does sodium butyrate stink, while sodium chloride does not?
Hydrogen chloride and sodium hydroxide are strong acids and bases, respectively. They react instantly and irreversibly with each other to form table salt and water. A strong acid is one that easily and immediately ionizes into H+ (a proton) and a counterion. Let's call it Z-. When the H and the Z want to get the hell away from each other – the case with hydrogen chloride – you have a strong acid.
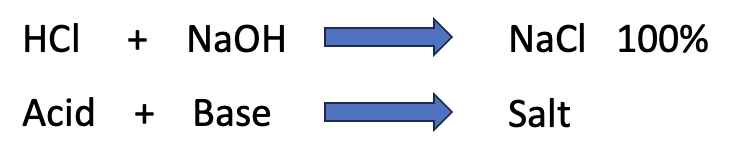
Figure 1 shows how sodium butyrate forms. The reaction isn’t the exciting part — the butyric acid is. Even if you don’t know it by name, you’ve definitely smelled or tasted it in one form or another.
By contrast, weak acids – butyric acid is one of them – are only partly ionized. In other words, H and Z may still hate each other, but a bit less so. (Perhaps H has money that Z wants to get its hands on.) When reacted with sodium hydroxide (another neutralization reaction), a small amount of the smelly butyric acid always lingers. This is indicated by the tiny backward arrow in Figure 2. In the presence of any water, there is an equilibrium, albeit one that greatly favors the salt form.
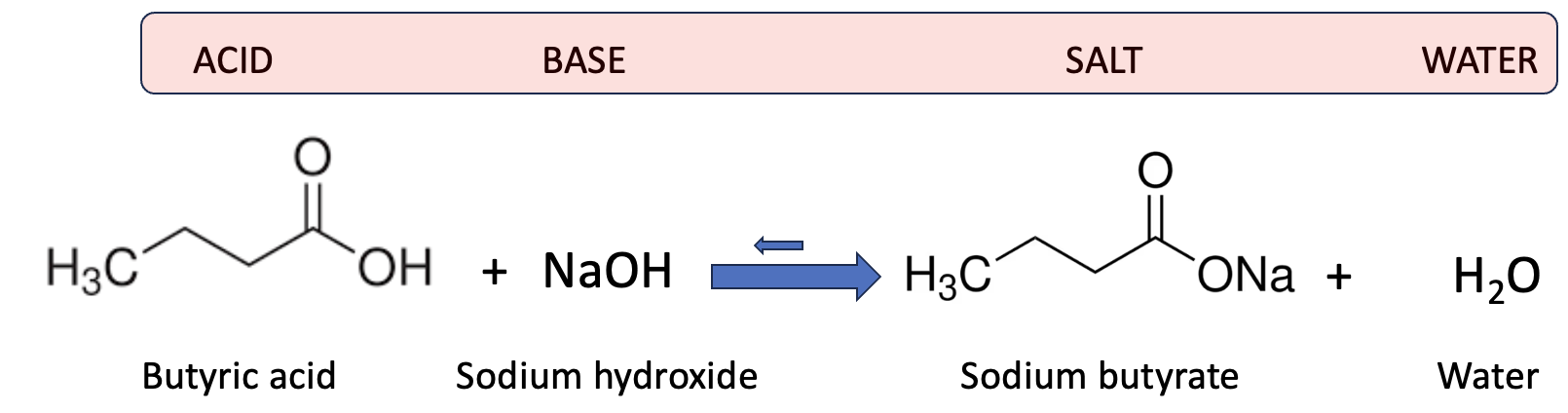
Figure 2. The neutralization of butyric acid by sodium hydroxide.
Depending on the pH, the equilibrium favors the salt, sodium butyrate, by a factor of 5,000 to 10,000 — meaning only about 0.02% of it exists as the free acid. That sounds trivial, but it’s not. Humans can detect butyric acid at concentrations as low as one part per billion, so even that microscopic fraction is enough to make an entire room smell like the bathroom in a scuzzy bar on St. Patrick's day. In other words, chemistry calls it “trace amounts”; your nose calls it a biohazard.
If you insist on numbers, that 0.02% works out to roughly 0.005 milligrams of butyric acid — just enough to stink up an entire room. One drop could perfume (if that’s the right word) anywhere from 500 to 5,000 rooms. That’s why vomit odor is so maddeningly persistent, especially in porous surfaces. Baking soda will neutralize some of it, converting it to the less offensive sodium butyrate, but equilibrium being equilibrium, a little bit of the foul stuff always hangs around. Luckily, it’s somewhat volatile (boiling point 163 °C), so with time—and open windows—it will eventually drift away.
Various descriptions of BA include the words:
- foul
- rancid
- ugly
- putrid
- It stinks of horrible things.
Why is it important?
Sorry, wrong article. The function of BA is complex and doesn't belong in this article. It involves signaling gene expression, energy generation, and inflammation. Research is testing its potential to help with cancer, metabolic problems, and brain diseases. Here's a review.
A Stinky Conclusion
It may seem odd that such an important chemical has such an off-putting odor, but this is not a coincidence. Because it’s found in decaying food, rancid fat, vomit, and feces, humans evolved to find the smell of butyric acid disgusting. This instinct helped our ancestors avoid spoiled or infectious material and is part of the body’s early “behavioral immune system” that prevents disease through avoidance.
So, next time you send Amazon $79.99 (free overnight delivery!) for a bottle of powdered puke capsules, remember that you are what you eat.
NOTE
[1] There is actually very little BA in the stomach. Most of it is found in the small intestine.



