Let's get something straight right away.
“For too long, some food producers have been feeding Americans petroleum-based chemicals without their knowledge or consent. These poisonous compounds offer no nutritional benefit and pose real, measurable dangers to our children’s health and development. That era is coming to an end."
Robert F. Kennedy Jr., Secretary of HHS, April 2025
"No, it's not."
Josh Bloom, chemist, whenever...
Without petroleum-based hydrocarbons, there would be no life on Earth. Let's explore.
Am I Just Being a Pedantic Pain in the A$$?
Part of this is true, but it's not the pedantry. But it's literally true that without the always-maligned "petroleum-based hydrocarbons," you wouldn't be reading this because I wouldn't have written it. I'm not exaggerating.
Like Secretary Kennedy, people get this wrong constantly. While there are numerous examples of these chemicals, I've picked out four. These examples should serve to debunk two myths.
Can't Live Without 'Em
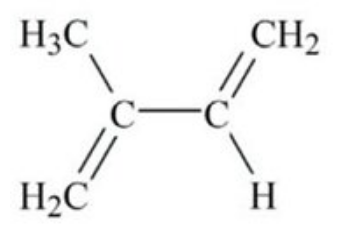
Ethylene, aka ethene
With the exception of methane, ethylene is the most volatile chemical to come from oil feedstocks. It becomes a gas at -155oF – not terribly different from Northern Florida last week. You will never encounter liquid ethylene outside a chemistry lab (and probably not one either).
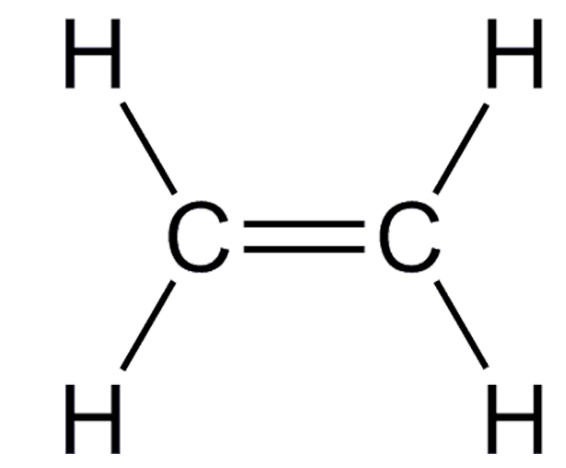
The chemical structure of ethylene.
Ethylene, which is produced by plants, is the primary ripening hormone of many fruits and also controls the spoilage and senescence (natural aging and breakdown) of plants (including vegetables). It is a near-universal plant hormone; without it, there would be no vegans. That's not all that troubling in itself, but there also wouldn't be any of us to be annoyed by them.
Since it's such an important molecule, let's examine the differences between the ethylene from plants and the ethylene from crude oil.
No, let's not
If I devoted my entire life to trying to explain to people that ethylene = ethylene, regardless of the source, I would fail miserably. People refuse to accept this, which is true for all other chemicals in the universe. So, no, I give up. I'm not going to waste our time blabbering about this.
Squalene
Squalene, which takes its name from the shark liver oil of Squalus (dogfish sharks), is a large and highly important hydrocarbon because of both its size (30 carbon atoms!) and its biological role. In the absence of squalene, the indispensable precursor of cholesterol, there would be no humans, animals, or Eagles fans.
Speaking of organisms with limited intellectual capacity, if not for cholesterol, animal cell membranes would be unstable and leaky — and animal life wouldn’t exist.
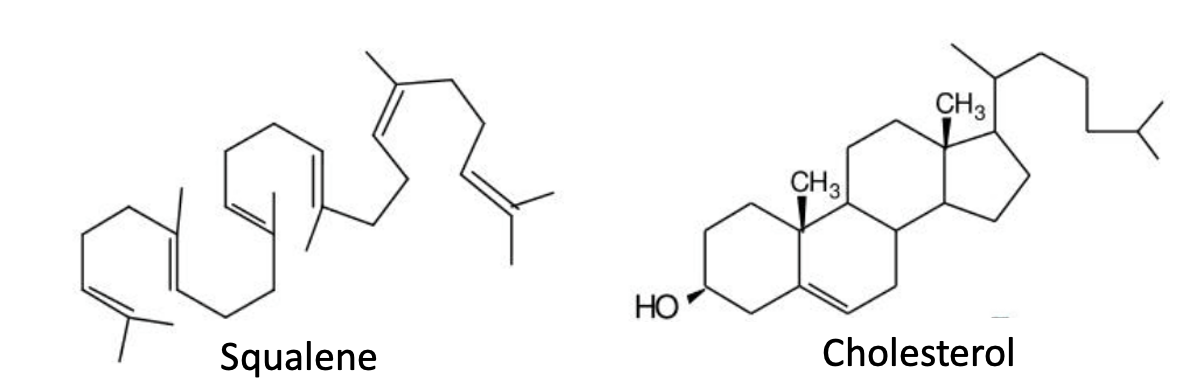
Figure 1. When squalene and cholesterol are drawn in the same conformation, it's easy to see that they are very similar, especially if you've been microdosing acid.
There are traces of squalene in crude oil, so it is technically an oil-based hydrocarbon, but, of course, this doesn't matter. (I don't know why I'm bothering to mention this. Again. I don't get paid by the word.)
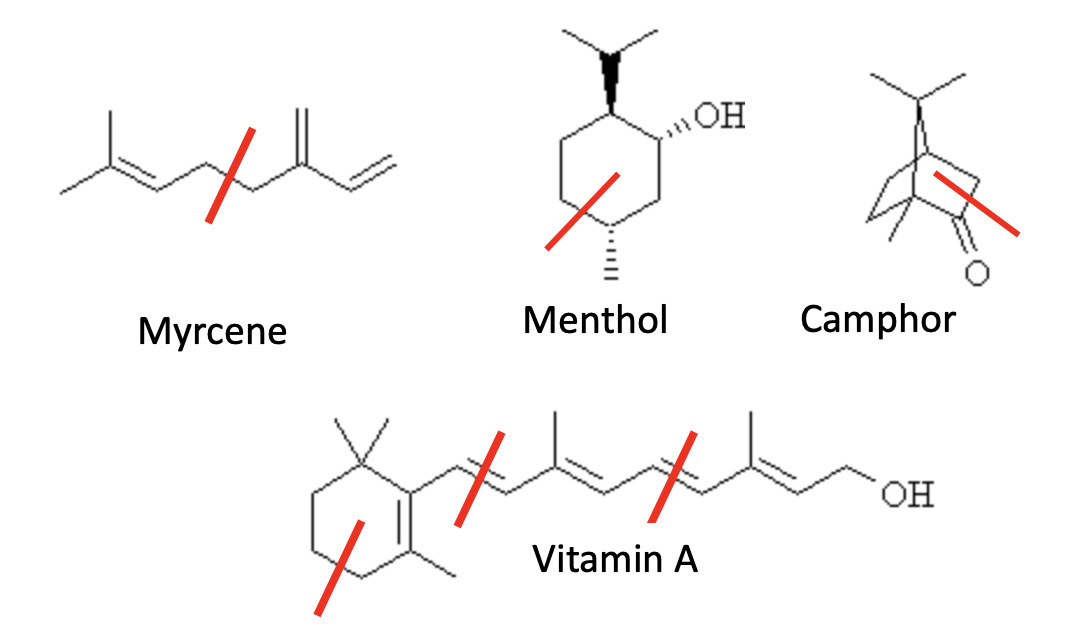
Isoprene
Beta-Carotene
β-Carotene, another hydrocarbon, is the orange pigment that gives carrots, sweet potatoes, and pumpkins their color, and it’s one of the most common dietary carotenoids on Earth (Figure 4).
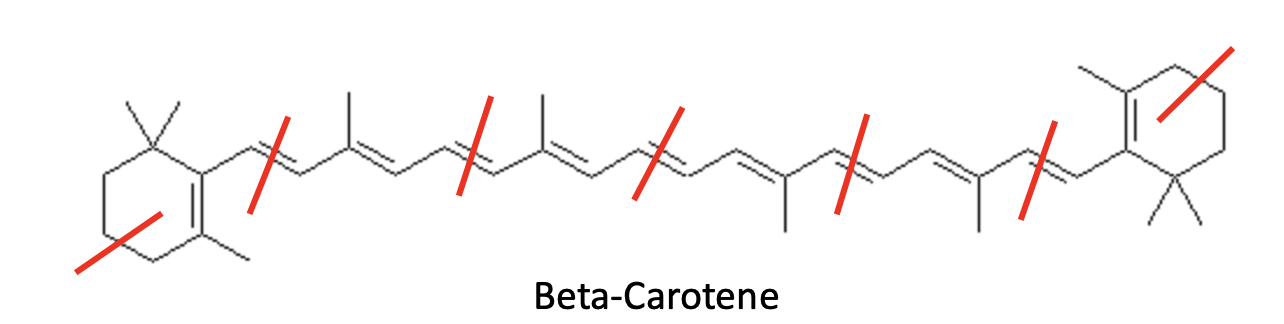
Figure 4. Beta-carotene, a tetraterpene, is a hydrocarbon built from eight isoprene units.
More importantly, β-carotene is a provitamin: your body converts it to Vitamin A, which is needed for vision, immune function, and staying alive in general. In other words, this “petroleum-like hydrocarbon” is not only natural — it’s literally a nutrient, and without it (or other vitamin A sources) you’d eventually go blind, get sick, and die. Among other things.
Bottom line
It should be obvious that these four chemicals–all naturally occurring hydrocarbons found in plants and in petroleum- are not only not harmful, but each is responsible for plant life, animal life, or both. So, when politicians, environmental know-nothings, and miscellaneous other non-scientists use the term "petrochemicals" to paint a particular chemical (or class of chemicals) as a poison, they are doing so either out of ignorance or malfeasance.
This ain't hard, folks. Even Eagles fans ought to get it.
NOTE:
[1] Terpenes are hydrocarbons made from repeating isoprene (C₅H₈) units — they contain only carbon and hydrogen. Terpenoids (aka isoprenoids) are modified terpenes that have been chemically altered — most often by adding oxygen (like alcohols, ketones, aldehydes) or by rearranging the carbon skeleton.