
As difficult as it is to believe, we are still having a debate about thimerosal, thanks to vaccine "skeptic" Robert F. Kennedy Jr., who is the head of HHS.
Kennedy and the ACIP [1] "experts" he selected are raising long-discredited concerns about thimerosal, aka "ethylmercury," a preservative that was falsely linked to autism [2].
I won't be delving into this controversial topic; others with far more expertise have discussed this in length. But the underlying confusion about "good" vs. "bad" mercury can be answered with some rather basic chemistry. An understanding of this chemistry should (but probably won't) defuse at least some of the overblown claims of neurotoxicity of the particular "form" of mercury that is used in multi-dose flu vaccines (and nowhere else). Nonetheless, here are some facts. These are not debatable.
All Mercury is not the same.
The word "mercury" is pretty much guaranteed to strike fear into the heart of much of the population. Sometimes this fear is justified; sometimes it is not. Here's a brief summary of 4 relevant forms of mercury. Some are scary, while others are not.
(For our ACSH video that explains the different types of mercury, click here: https://tinyurl.com/5ewejz3u)
- Elemental mercury (Hgo)
Elemental mercury, aka "quicksilver," is one of the two elements that are liquids at room temperature [3], the other being bromine. Although it is chemically unreactive, most mercury found on Earth is in the form of cinnabar, a bright red to brown crystalline mineral composed of mercury and sulfur. The pairing of mercury and sulfur is not a coincidence. For reasons that are beyond the scope of this article, the two elements form a very strong bond. Mercury simply loves sulfur.
Despite its fearsome reputation, elemental mercury (Hg⁰) is less toxic than most people think, at least in liquid form. It was even used in ancient China as an “immortality elixir.” When swallowed, liquid mercury is poorly absorbed by the gastrointestinal tract; however, a small portion is converted into mercury chloride, a far more toxic and readily absorbed compound. The real danger lies in mercury vapor: it is readily inhaled, absorbed through the lungs, and transported to the brain and central nervous system. Chronic exposure—even to small amounts—can result in chronic mercury poisoning, historically known as “mad hatter syndrome.”
Let's pause for a story.
2. Mercury (II) salts (inorganic)
Soluble inorganic mercury+2 salts are deadly neurotoxins. Examples include mercury (II) chloride and nitrate. There are no medical uses for these chemicals, although they were used in the 17th-19th centuries as a laxative, reportedly by President Lincoln (they made him cranky). Mercury salts should be avoided, which isn't all that difficult since they have no known medical use, approved or otherwise.
3. Methylmercury (organic mercury)
Methylmercury, an organomercury compound [3], is largely responsible for the confusion surrounding mercury in vaccines. While it sounds similar to ethylmercury, aka thimerosal, the preservative used in some flu vaccines , the two differ significantly, particularly in how they are metabolized and excreted. Methylmercury is poorly eliminated from the body and accumulates in tissues, especially the brain. More on this below.
![]()
Figure 1. The chemical structure of methylmercury. Although commonly referred to as “methylmercury,” the compound exists in a partially ionic form, such as methylmercury chloride, with a +1 charge on the mercury atom balanced by a counterion, like chloride, which is functionally similar to a sodium salt.
Its chemical stability, lipid solubility, and ability to cross the blood-brain barrier make methylmercury especially dangerous. It was once used topically to treat skin infections, but this practice was abandoned decades ago due to absorption through the skin, leading to systemic neurotoxicity.
Methylmercury, which is the form of mercury that accumulates in fish, has NEVER been used as a vaccine preservative. (If you hear otherwise from a vaccine skeptic, they are dead wrong.)
4. Ethylmercury, aka Thimerosal
The difference between ethylmercury and methylmercury may only be the letter "M," but pharmacologically, it's night and day (Figure 2).
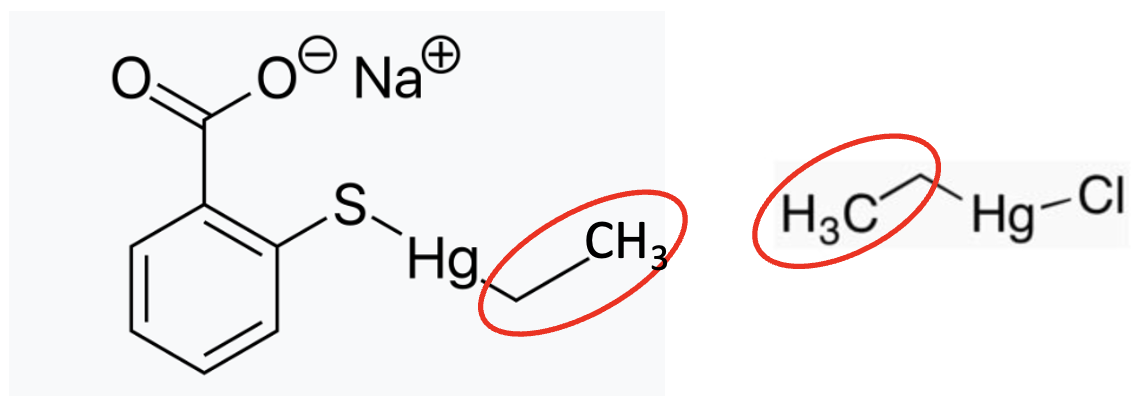
Figure 2. Although thimerosal (left) is commonly called ethylmercury (right), this is a "nickname" for ethyl(2-mercaptobenzoato-(2-)-O,S) mercurate(1-) sodium salt, if you prefer. Note that the thimerosal contains (and breaks down to) ethylmercury. The red ovals indicate the ethyl groups.
To understand the significant differences in toxicity between methylmercury and ethylmercury, it is necessary to examine the metabolism of each. These guys are so similar looking. How different can they really be? Plenty.
The half-life of methylmercury in humans is about two months. For ethylmercury, it's about one week. The result? Methylmercury accumulates in the body while ethylmercury is cleared. Figure 3 puts this in perspective.
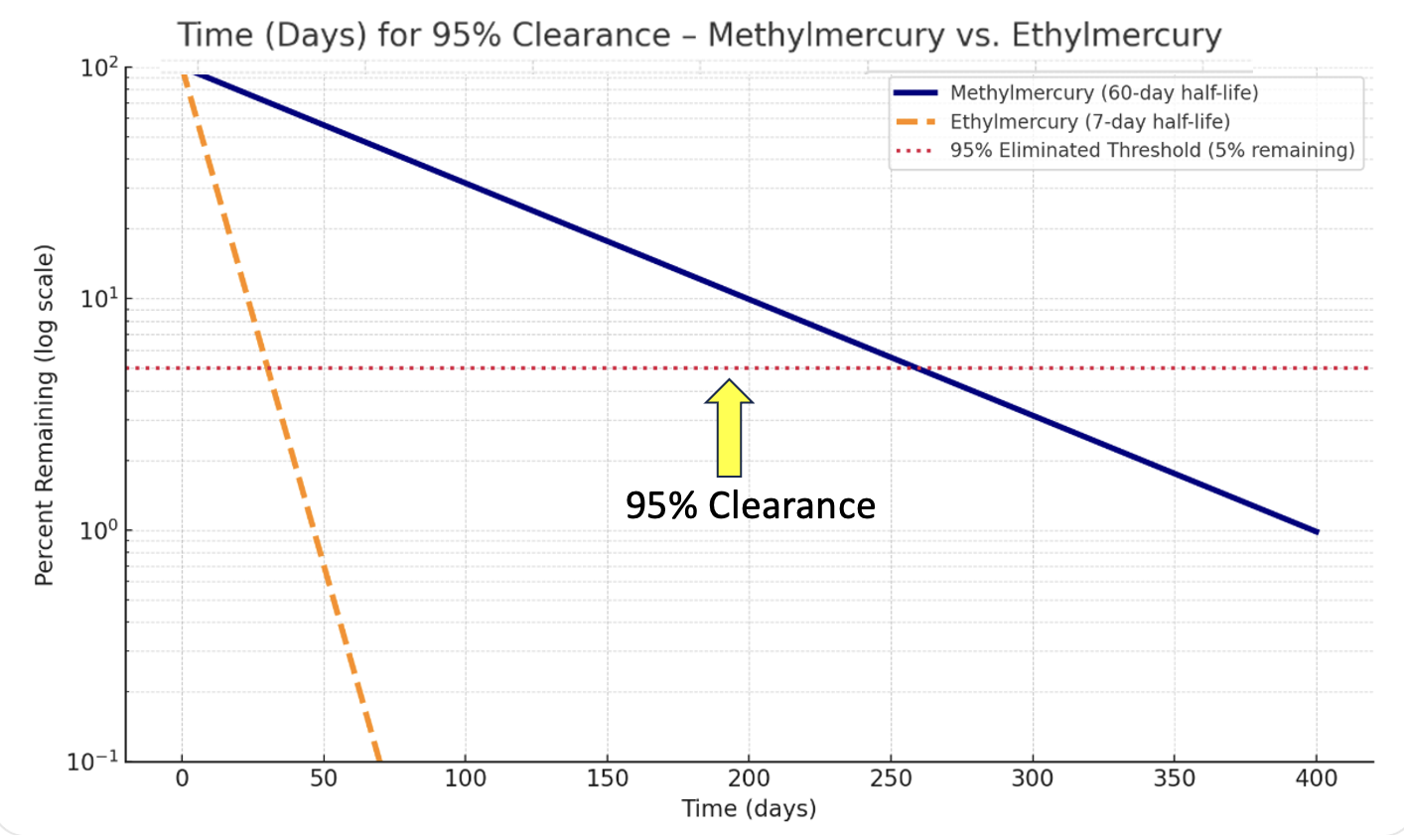
Figure 3. Modeled clearance of methylmercury (blue) versus ethylmercury (orange). Note the rapid elimination of ethylmercury compared to the months-long persistence of methylmercury.
Why? This requires a little chemistry.
When ethylmercury enters the bloodstream ("packaged" as thimerosal), the compound is rapidly dealkylated (ethyl group is removed) by a common metabolic pathway called oxidative dealkylation. That means the ethyl group is stripped away, leaving behind inorganic mercury (Hg²⁺) and a two-carbon fragment that is oxidized to acetaldehyde and then acetic acid, similar to the way that ethanol is metabolized. By contrast, the oxidative metabolism of methylmercury is slower for reasons that are beyond the scope of this article. This is why methylmercury persists in the body for much longer. (It’s an imperfect analogy, but similar to why methanol is more toxic than ethanol.)
Ignorance isn't bliss. It's just ignorance.
So let’s stop pretending that all forms of mercury are interchangeable—or equally dangerous. The continued fearmongering over thimerosal ignores basic toxicology and serves only to undermine trust in vaccines. Ethylmercury is not methylmercury. One clears in days; the other persists for months. If we’re going to have public debates about medicine, they should at least be grounded in chemistry. Otherwise, we’re just poisoning the conversation.
NOTES:
[1] The ACIP (Advisory Committee on Immunization Practices) is a CDC-affiliated panel of medical and (supposedly) public health experts that provides guidance on vaccine schedules and safety.
[2] When thimerosal was removed from nearly all childhood vaccines starting around 2001, autism rates continued to rise. So much for that theory? So, how did anti-vaxxers respond? They moved the goalposts and started blaming...pretty much everything else in the vaccine.
[3] Organometallic compounds are "normal" organic (carbon-containing) molecules that have a carbon atom bonded to a metal. Many of them are highly reactive.



