
It's no secret that I'm not a big fan of Tylenol. So, when someone asked me about extended-release Tylenol (aka Tylenol 8 Hour), I initially wrote it off as just another marketing scheme to sell more acetaminophen. Is this fair?
Yes and no, because what’s more satisfying than a firm “maybe”?
Or if that’s too vague, let’s go with the gold standard of medical hedging: theoretically.
Six of one...
One regular-strength Tylenol pill contains 325 mg of acetaminophen. The FDA maximum permitted dose is 4,000 mg per day [1]. Johnson & Johnson [2] directions are a bit more conservative: two pills or capsules every 4-6 hours with no more than 10 pills in one day.
(Note: J&J also sells Extra-Strength Tylenol, a larger dose (500 mg per pill) of the same drug, which is limited to 6 pills per day. How clever.)
Extended release: more of the same?
Yet another acetaminophen product – Tylenol 8 Hour – is offered by J&J. Its alleged advantage is a longer duration of action. You take a double dose (two 650 mg pills) half as often, hence the 8-Hour moniker. This would seem to be a remedy for laziness and not much else. But, that isn't fair. Extended-release drugs can offer substantial advantages over their fast-release counterparts. Here are a few examples.
Time-release (also called extended-release or sustained-release) drug formulations offer several key advantages over their immediate-release counterparts. One of the most significant benefits is improved dosing convenience and patient compliance. Instead of needing to take a medication every 4–6 hours, a patient may only need to take a time-release version once or twice daily. This simplified regimen helps reduce missed doses and, more importantly, maintains more consistent drug levels in the bloodstream, which is especially important for chronic conditions such as hypertension, pain management, and some psychiatric disorders.
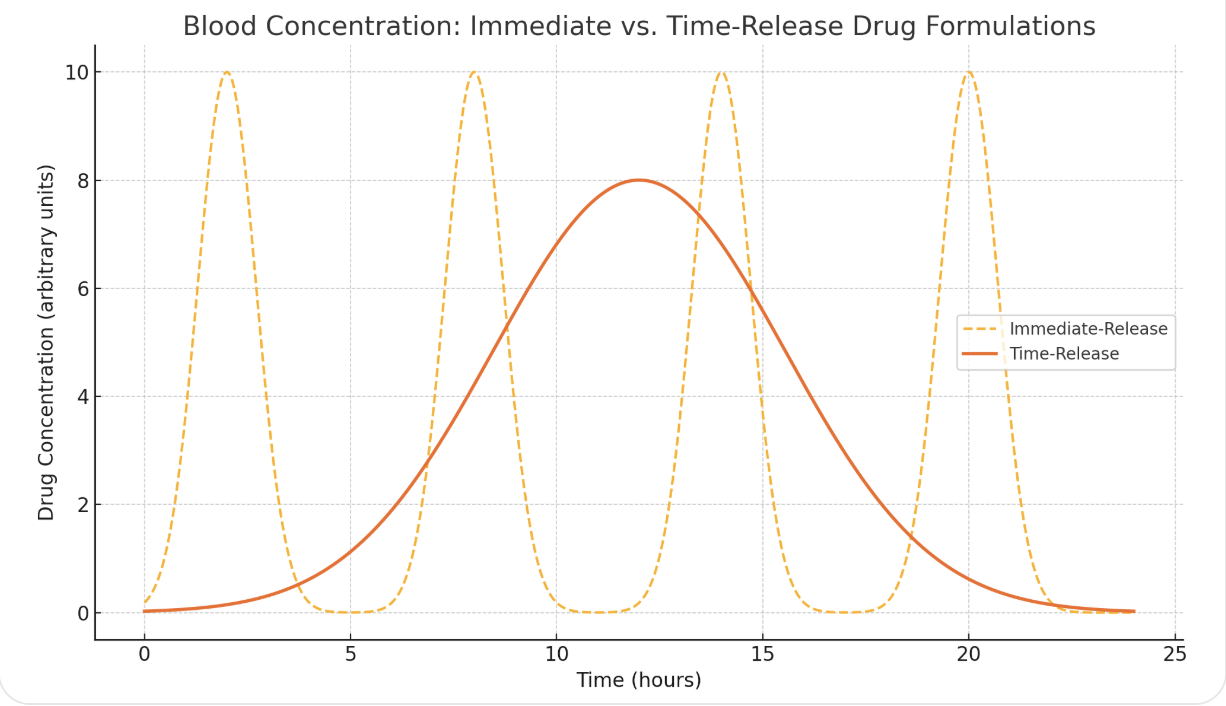
Figure 1. An illustration of the difference in blood levels in 4x/per day dosing and once daily.
Another major advantage is smoother pharmacokinetics—that is, a more stable concentration of the drug in the body over time (Figure 1). Immediate-release formulations tend to produce peaks and troughs: drug levels spike after dosing and then decline rapidly. These fluctuations can lead to side effects at high concentrations and reduced efficacy (and side effects) at low ones. Time-release formulations avoid these extremes by releasing the active ingredient gradually, minimizing side effects and improving overall therapeutic outcomes. For drugs with short half-lives or narrow therapeutic windows, sustained delivery can make a meaningful difference in both safety and effectiveness.
J&J accomplishes this by smushing together (technical term) two layers of Tylenol. The first layer dissolves quickly so that your non-relief can begin quickly, while inside lurks another dose that will start to dissolve after the first one is gone, ensuring you of 8 hours of continuous discomfort!
How many Tylenol pills can fit on the head of a pin?
Now, we're entering existential territory: If someone creates a superior form of an inferior, possibly even useless, drug, is this progress? This is just my opinion, but J&J's recommendation for you to swallow a more expensive but marginally effective pill less frequently is really asking you to swallow a lot more.
NOTES:
[1] Tylenol is currently sold by Kenvue Inc., a company that was spun off from Johnson & Johnson in 2023. I don't know what a "Kenvue" is, so I'm gonna stick with Johnson & Johnson.
[2] Rumor has it that the FDA lowered the maximum dose of APAP from 4 to 3 grams per day. This is incorrect. Manufacturers took that upon themselves. The agency lowered the maximum amount of APAP that can be included in a prescription medication (such as Percocet) to 325 mg per dose in 2011.



