Upon chemiexcitation via exergonic oxalate ester decomposition, the dye’s π→π* transition populates its lowest vibrationally relaxed singlet excited manifold (S₁,v=0), wherein nonradiative internal conversion ceases prior to spontaneous radiative deactivation, yielding photon emission to the S₀ ground state.
Hope you're happy now.
Pumpkin Spice
Definitely an acquired taste (as in: "You acquire it and leave me alone." It's a witches’ brew of chemicals. Here are some of them.
- trans-Cinnamaldehyde – unsurprisingly, it is the chemical that gives cinnamon its signature scent. No, it has not undergone gender-affirming surgery, but it would be understandable if its inferior isomer, cis-cinnamaldehyde, did so. The unfortunate isomer lacks the sweet smell and is not welcome at the dinner table.
- Eugenol – Found in cloves. This is the yicky stuff that dentists pack into your half-excavated tooth during the beloved root canal experience. It calms the nerves in the tooth.
- Zingiberene – The best name of the bunch. It gives ginger its odor and flavor. It does not contribute to ginger's antiemetic properties, but its cousin, Zingerone, does. There are also claims of benefits for multiple human health conditions. It is not found in these...

Raspberry Zingers. Blech. Don't they look like a biopsy specimen?
Ammonium Dichromate – The “Volcano Chemical”
Although ammonium dichromate ((NH₄)₂Cr₂O₇) was once used in Halloween science displays, it’s now been given the boot for safety reasons (please). You may have seen the classic “volcano experiment,” where it decomposes to produce bright orange flames, green ash, and smoke — looking like something bubbling out of a witch’s cauldron. Or Godzilla's stomach contents following eating a Butterfinger.
Although you won't see it in science demos anymore, here’s a nice YouTube video of ammonium dichromate burning — which it does with cheerful ferocity. Friendly advice: do not assume that nose hair won’t burn.
Dry Ice
As long as we're in the mood to blow stuff up, it would be wrong to ignore the sheer terror that can be dispensed by using a few chunks of dry ice. I know this because I've been the perpetrator of CO₂ terror. First, a little about the stuff. Dry ice (solid CO₂) [3] is a Halloween staple. It sublimes at –78.5 °C, producing that iconic white, ground-hugging fog that you've seen many times, especially in a haunted house.
Drop it into a bowl of punch, and it immediately begins to vaporize, turning the bowl into something that looks like it could reanimate a corpse. The “fog” isn’t really smoke at all; it’s just condensed water vapor riding along with the escaping CO₂ gas. CO₂ itself is invisible.
It’s all quite harmless if you remember not to touch it. The same temperature that makes it look like the gates of hell are opening will also give you an instant frostbite souvenir if you pick it up bare-handed. Also, be careful not to drop it into your underwear. Whether you choose to drop it into someone else's underwear is up to you and your desire to avoid prison time—purely a matter of risk and benefit.
Extra stuff: How to scare the hell out of a fellow chemist using dry ice.
- Find a latex pipette bulb.
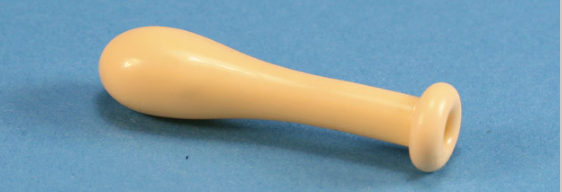
2. Put in a few chunks of dry ice and clamp the end.
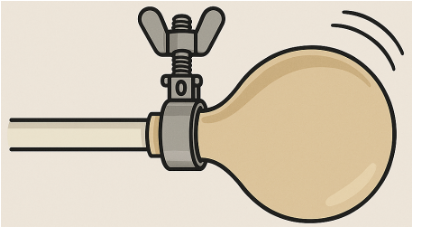
3. Hide it under the victim's desk. Yes, I have really done this. And cover your ears.
4. Await results.
Pipette bulb-induced terror.
And it may be more audible than you think...
Warning: Do not underestimate the power of the CO2 pipette bulb device.
Bottom Spooky Line
Halloween is proof that the line between "chemical curiosity" and "crime scene" is as clear as a smoky haunted house. It's wise to stay on the right side of that line (said a true hypocrite who did anything but).
NOTES:
[1] The earwax comment isn't as nuts as it seems. One of the ingredients in evil candy corn is carnauba wax. And wax is wax. Does anyone know how to define it?
[2] I can say this because they were stupid enough to hire me. American Cyanamid was the parent company of Lederle Laboratories, the chuckheads who hired me long ago. If it wasn't the worst pharmaceutical company on earth, it was damn close. Later, Cyanamid (great idea having "cyan" in the name of a drug company, right?) was taken over by American Home Products (AHP), which sold everything from junk food to horse piss (The hormone replacement Premarin is derived from PREgnant MARe UrINe, in other words, horse piss.) Wyeth Ayerst, perhaps not as dumb as Lederle but close, was the pharmaceutical arm of AHP. Later, the company changed its name so as to sound like it wasn't selling Ajax, to Wyeth, focusing solely on the development of novel, life-saving drugs, something it did exceedingly poorly (See: Fen-Phen).
[3] Carbon dioxide can exist in a liquid form but you've never seen it. It occurs only under certain conditions: -70°F and 5 atmospheres of pressure, not terribly different from an average winter day in Buffalo.